Traditional and Modern Biomedical Prospecting: Part II-the Benefits: Approaches for a Sustainable Exploitation of Biodiversity (Secondary Metabolites and Biomaterials from Sponges)
- PMID: 15480439
- PMCID: PMC516461
- DOI: 10.1093/ecam/neh030
Traditional and Modern Biomedical Prospecting: Part II-the Benefits: Approaches for a Sustainable Exploitation of Biodiversity (Secondary Metabolites and Biomaterials from Sponges)
Abstract
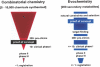
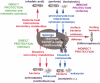
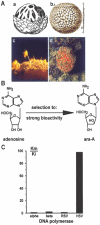
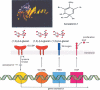
The progress in molecular and cell biology has enabled a rational exploitation of the natural resources of the secondary metabolites and biomaterials from sponges (phylum Porifera). It could be established that these natural substances are superior for biomedical application to those obtained by the traditional combinatorial chemical approach. It is now established that the basic structural and functional elements are highly conserved from sponges to the crown taxa within the Protostomia (Drosophila melanogaster and Caenorhabditis elegans) and Deuterostomia (human); therefore, it is obvious that the molecular etiology of diseases within the metazoan animals have a common basis. Hence, the major challenge for scientists studying natural product chemistry is to elucidate the target(s) of a given secondary metabolite, which is per se highly active and selective. After this step, the potential clinical application can be approached. The potential value of some selected secondary metabolites, all obtained from sponges and their associated microorganisms, is highlighted. Examples of compounds that are already in medical use (inhibition of tumor/virus growth [arabinofuranosyl cytosine and arabinofuranosyl adenine]), or are being considered as lead structures (acting as cytostatic and anti-inflammatory secondary metabolites [avarol/avarone], causing induction of apoptosis [sorbicillactone]) or as prototypes for the interference with metabolic pathways common in organisms ranging from sponges to humans (modulation of pathways activated by fungal components [aeroplysinin], inhibition of angiogenesis [2-methylthio-1,4-napthoquinone], immune modulating activity [FK506]) are discussed in this study. In addition, bioactive proteins from sponges are listed (antibacterial activity [pore-forming protein and tachylectin]). Finally, it is outlined that the skeletal elements-the spicules-serve as blueprints for new biomaterials, especially those based on biosilica, which might be applied in biomedicine. These compounds and biomaterials have been isolated/studied by members of the German Center of Excellence BIOTECmarin. The goal for the future is to successfully introduce some of these compounds in the treatment of human diseases in order to raise the public awareness on the richness and diversity of natural products, which should be sustainably exploited for human benefit.
Figures



References
-
- Bringmann G, Lang G, Mühlbacher J, Schaumann K, Steffens S, Rytik PG, et al. Sorbicillactone A, a structurally unprecedented bioactive novel-type alkaloid from a sponge-derived fungus. In: Müller WEG, editor. Sponges (Porifera). Marine Molecular Biotechnology. Berlin: Springer; 2003. pp. 231–253. - PubMed
-
- Müller WEG, Klemt M, Thakur NL, Schröder HC, Aiello A, D'Esposito M, et al. Molecular/chemical ecology in sponges: evidence for an adaptive antibacterial response in Suberites domuncula. Mar Biol. 2003;144:19–29.
-
- Perović-Ottstadt S, Adell T, Proksch P, Wiens M, Korzhev M, Gamulin V, et al. A (1→3)-b-D-glucan recognition protein from the sponge Suberites domuncula: mediated activation of fibrinogen-like protein and epidermal growth factor gene expression. Eur J Biochem. 2004;271:1924–1937. - PubMed
-
- Müller WEG, Wiens M, Adell T, Gamulin V, Schröder HC, Müller IM. Bauplan of the Urmetazoa: the basis of the genetic complexity of Metazoa using the siliceous sponges [Porifera] as living fossils. Intern Rev Cytol. 2004;235:53–92. - PubMed
-
- Breter HJ, Grebenjuk VA, Skorokhod A, Müller WEG. Approaches for a sustainable use of the bioactive potential in sponges: analysis of gene clusters, differential display of mRNA and DNA chips. In: Müller WEG, editor. Sponges (Porifera). Marine Molecular Biotechnology. Berlin: Springer; 2003. pp. 199–230. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

