Activation of ryanodine receptors induces calcium influx in a neuroblastoma cell line lacking calcium influx factor activity
- PMID: 15482258
- PMCID: PMC1134793
- DOI: 10.1042/BJ20040900
Activation of ryanodine receptors induces calcium influx in a neuroblastoma cell line lacking calcium influx factor activity
Abstract
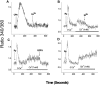
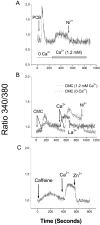
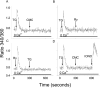
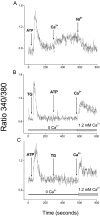
We have further characterized the Ca2+ signalling properties of the NG115-401L (or 401L) neuroblastoma cell line, which has served as an important cell line for investigating SOC (store-operated channel) influx pathways. These cells possess an unusual Ca2+ signalling phenotype characterized by the absence of Ca2+ influx when Ca2+ stores are depleted by inhibitors of SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase). Previous studies found that Ca2+-store depletion does not produce a CIF (Ca2+ influx factor) activity in 401L cells. These observations have prompted the question whether 401L cells possess the signalling machinery that permits non-voltage-gated Ca2+ influx to occur. We tested the hypothesis that ryanodine-sensitive Ca2+ pools and activation of RyRs (ryanodine receptors) constitute a signalling pathway capable of inducing Ca2+ influx in 401L cells. We found that 401L cells express mRNA for RyR1 and RyR2 and that RyR activators induced Ca2+ release. Activation of RyRs robustly couples with Ca2+ influx responses in 401L cells, in sharp contrast with absence of Ca2+ influx when cells are treated with SERCA inhibitors. Thus it is clear that 401L cells, despite lacking depletion-induced Ca2+ influx pathways, express the functional components of a Ca2+ influx pathway under the control of RyR function. These findings further support the importance of the 401L cell line as an important cell phenotype for deciphering Ca2+ influx regulation.
Figures
Similar articles
-
Biochemical characterization, distribution and phylogenetic analysis of Drosophila melanogaster ryanodine and IP3 receptors, and thapsigargin-sensitive Ca2+ ATPase.J Cell Sci. 2003 Jun 15;116(Pt 12):2483-94. doi: 10.1242/jcs.00455. J Cell Sci. 2003. PMID: 12766186
-
Ca2+-induced Ca2+ release by activation of inositol 1,4,5-trisphosphate receptors in primary pancreatic beta-cells.Cell Calcium. 2004 Jul;36(1):1-9. doi: 10.1016/j.ceca.2003.11.004. Cell Calcium. 2004. PMID: 15126051
-
2-Aminoethoxydiphenyl borate (2-APB) stimulates a conformationally coupled calcium release pathway in the NG115-401L neuronal cell line.Neuropharmacology. 2006 Apr;50(5):532-9. doi: 10.1016/j.neuropharm.2005.10.011. Epub 2005 Dec 2. Neuropharmacology. 2006. PMID: 16325870
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
-
Ryanodine receptor calcium release channels.Physiol Rev. 2002 Oct;82(4):893-922. doi: 10.1152/physrev.00013.2002. Physiol Rev. 2002. PMID: 12270947 Review.
Cited by
-
Impairment of Tricarboxylic Acid Cycle (TCA) Cycle in Alzheimer's Disease: Mechanisms, Implications, and Potential Therapies.Aging Dis. 2025 May 29;16(5):2553-2574. doi: 10.14336/AD.2025.0472. Aging Dis. 2025. PMID: 40479573 Free PMC article. Review.
-
Novel role for STIM1 as a trigger for calcium influx factor production.J Biol Chem. 2008 May 23;283(21):14524-31. doi: 10.1074/jbc.M709575200. Epub 2008 Mar 12. J Biol Chem. 2008. PMID: 18337241 Free PMC article.
-
Genome wide expression analysis in HPV16 cervical cancer: identification of altered metabolic pathways.Infect Agent Cancer. 2007 Sep 6;2:16. doi: 10.1186/1750-9378-2-16. Infect Agent Cancer. 2007. PMID: 17822553 Free PMC article.
-
The actin cytoskeleton differentially regulates NG115-401L cell ryanodine receptor and inositol 1,4,5-trisphosphate receptor induced calcium signaling pathways.Biochem Biophys Res Commun. 2009 Feb 6;379(2):594-9. doi: 10.1016/j.bbrc.2008.12.138. Epub 2009 Jan 4. Biochem Biophys Res Commun. 2009. PMID: 19126405 Free PMC article.
-
Ryanodine receptor 1-mediated Ca2+ signaling and mitochondrial reprogramming modulate uterine serous cancer malignant phenotypes.J Exp Clin Cancer Res. 2022 Aug 11;41(1):242. doi: 10.1186/s13046-022-02419-w. J Exp Clin Cancer Res. 2022. PMID: 35953818 Free PMC article.
References
-
- Berridge M. J., Lipp P., Bootman M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. - PubMed
-
- Venkatachalam K., van Rossum D. B., Patterson R. L., Ma H. T., Gill D. L. The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 2002;4:E263–E272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

