Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways
- PMID: 15483113
- PMCID: PMC6730057
- DOI: 10.1523/JNEUROSCI.3065-04.2004
Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways
Abstract
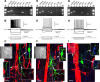
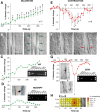
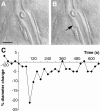
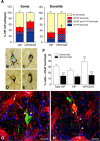
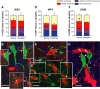
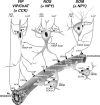
The role of interneurons in neurovascular coupling was investigated by patch-clamp recordings in acute rat cortical slices, followed by single-cell reverse transcriptase-multiplex PCR (RT-mPCR) and confocal observation of biocytin-filled neurons, laminin-stained microvessels, and immunodetection of their afferents by vasoactive subcortical cholinergic (ACh) and serotonergic (5-HT) pathways. The evoked firing of single interneurons in whole-cell recordings was sufficient to either dilate or constrict neighboring microvessels. Identification of vasomotor interneurons by single-cell RT-mPCR revealed expression of vasoactive intestinal peptide (VIP) or nitric oxide synthase (NOS) in interneurons inducing dilatation and somatostatin (SOM) in those eliciting contraction. Constrictions appeared spatially restricted, maximal at the level of neurite apposition, and were associated with contraction of surrounding smooth muscle cells, providing the first evidence for neural regulation of vascular sphincters. Direct perfusion of VIP and NO donor onto the slices dilated microvessels, whereas neuropeptide Y (NPY) and SOM induced vasoconstriction. RT-PCR analyses revealed expression of specific subtypes of neuropeptide receptors in smooth muscle cells from intracortical microvessels, compatible with the vasomotor responses they elicited. By triple and quadruple immunofluorescence, the identified vasomotor interneurons established contacts with local microvessels and received, albeit to a different extent depending on interneuron subtypes, somatic and dendritic afferents from ACh and 5-HT pathways. Our results demonstrate the ability of specific subsets of cortical GABA interneurons to transmute neuronal signals into vascular responses and further suggest that they could act as local integrators of neurovascular coupling for subcortical vasoactive pathways.
Figures

References
-
- Abounader R, Hamel E (1997) Associations between Neuropeptide Y nerve terminals and intraparenchymal microvessels in rat and human cerebral cortex. J Comp Neurol 388: 444-453. - PubMed
-
- Abounader R, Elhusseiny A, Cohen Z, Olivier A, Stanimirovic D, Quirion R, Hamel E (1999) Expression of neuropeptide Y receptors mRNA and protein in human brain vessels and cerebromicrovascular cells in culture. J Cereb Blood Flow Metab 19: 155-163. - PubMed
-
- Bayraktar T, Staiger JF, Acsady L, Cozzari C, Freund TF, Zilles K (1997) Co-localization of vasoactive intestinal polypeptide, gamma-aminobutyric acid and choline acetyltransferase in neocortical interneurons of the adult rat. Brain Res 757: 209-217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
