Alpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse
- PMID: 15483118
- PMCID: PMC6730050
- DOI: 10.1523/JNEUROSCI.3184-04.2004
Alpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse
Abstract
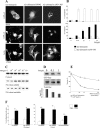
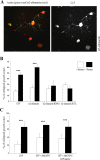
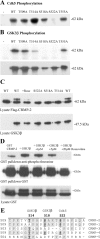
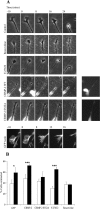
Neurite outgrowth is influenced by positive and negative signals that include the semaphorins, an important family of axonal outgrowth inhibitors. Here we report that the Rac GTPase activating protein (GAP)alpha2-chimaerin is involved in Semaphorin 3A (Sema 3A) signaling. In dorsal root ganglion neurons, Sema 3A-induced growth cone collapse was inhibited by alpha2-chimaerin mutated to eliminate GAP activity or interaction with phosphotyrosine. Activation of alpha2-chimaerin by phorbol ester caused growth cone collapse. Active alpha2-chimaerin interacts with collapsin response mediator protein-2 (CRMP-2) and cyclin-dependent kinase (Cdk) 5/p35 kinase through its SH2 and GAP domains, respectively. Cdk5 phosphorylates CRMP-2 at serine 522, possibly facilitating phosphorylation of serine 518 and threonine 514 by glycogen synthase kinase 3beta (GSK3beta), a kinase previously implicated in Sema 3A signaling. Phosphorylation of CRMP-2 serine 522 was essential for Sema 3A-induced growth cone collapse, which is dependent on Cdk5 but not Rho kinase activity. alpha2-chimaerin, like CRMP-2, can associate with the Sema 3A receptor. These results indicate that active alpha2-chimaerin Rac GAP, Cdk5/p35, and its substrate CRMP-2, are implicated in the dynamics of growth cone guidance initiated through Sema 3A signaling.
Figures




References
-
- Ahmed S, Lee J, Kozma R, Best A, Monfries C, Lim L (1993) A novel functional target for tumor-promoting phorbol esters and lysophosphatidic acid. The p21rac-GTPase activating protein n-chimaerin. J Biol Chem 268: 10709-10712. - PubMed
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I (2001) Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 4: 367-373. - PubMed
-
- Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshima Y, Kaibuchi K (2000) Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem 275: 23973-23980. - PubMed
-
- Caloca MJ, Fernandez N, Lewin NE, Ching D, Modali R, Blumberg PM, Kazanietz MG (1997) Beta2-chimaerin is a high affinity receptor for the phorbol ester tumor promoters. J Biol Chem 272: 26488-26496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
