Physiological and morphological characterization of dentate granule cells in the p35 knock-out mouse hippocampus: evidence for an epileptic circuit
- PMID: 15483119
- PMCID: PMC6730067
- DOI: 10.1523/JNEUROSCI.2943-04.2004
Physiological and morphological characterization of dentate granule cells in the p35 knock-out mouse hippocampus: evidence for an epileptic circuit
Abstract
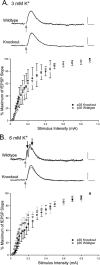
There is a high correlation between pediatric epilepsies and neuronal migration disorders. What remains unclear is whether there are intrinsic features of the individual dysplastic cells that give rise to heightened seizure susceptibility, or whether these dysplastic cells contribute to seizure activity by establishing abnormal circuits that alter the balance of inhibition and excitation. Mice lacking a functional p35 gene provide an ideal model in which to address these questions, because these knock-out animals not only exhibit aberrant neuronal migration but also demonstrate spontaneous seizures. Extracellular field recordings from hippocampal slices, characterizing the input-output relationship in the dentate, revealed little difference between wild-type and knock-out mice under both normal and elevated extracellular potassium conditions. However, in the presence of the GABA(A) antagonist bicuculline, p35 knock-out slices, but not wild-type slices, exhibited prolonged depolarizations in response to stimulation of the perforant path. There were no significant differences in the intrinsic properties of dentate granule cells (i.e., input resistance, time constant, action potential generation) from wild-type versus knock-out mice. However, antidromic activation (mossy fiber stimulation) evoked an excitatory synaptic response in over 65% of granule cells from p35 knock-out slices that was never observed in wild-type slices. Ultrastructural analyses identified morphological substrates for this aberrant excitation: recurrent axon collaterals, abnormal basal dendrites, and mossy fiber terminals forming synapses onto the spines of neighboring granule cells. These studies suggest that granule cells in p35 knock-out mice contribute to seizure activity by forming an abnormal excitatory feedback circuit.
Figures





Comment in
-
Are altered excitatory synapses found in neuronal migration disorders?Epilepsy Curr. 2005 Sep-Oct;5(5):171-3. doi: 10.1111/j.1535-7511.2005.00054.x. Epilepsy Curr. 2005. PMID: 16175215 Free PMC article. No abstract available.
Similar articles
-
Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats.J Neurophysiol. 2000 Feb;83(2):693-704. doi: 10.1152/jn.2000.83.2.693. J Neurophysiol. 2000. PMID: 10669485
-
Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth.J Neurophysiol. 1999 Apr;81(4):1645-60. doi: 10.1152/jn.1999.81.4.1645. J Neurophysiol. 1999. PMID: 10200201
-
Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats.J Neurophysiol. 1998 Jan;79(1):418-29. doi: 10.1152/jn.1998.79.1.418. J Neurophysiol. 1998. PMID: 9425210
-
Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system.Prog Brain Res. 2007;163:541-63. doi: 10.1016/S0079-6123(07)63029-5. Prog Brain Res. 2007. PMID: 17765737 Review.
-
Functional and morphological changes in the hippocampal neuronal circuits associated with epileptic seizures.Epilepsia. 2002;43 Suppl 9:44-9. doi: 10.1046/j.1528-1157.43.s.9.11.x. Epilepsia. 2002. PMID: 12383280 Review.
Cited by
-
The Expression Alteration of BC1 RNA and its Interaction with Eukaryotic Translation Initiation Factor eIF4A Post-Status Epilepticus.Neurochem Res. 2018 Jul;43(7):1328-1338. doi: 10.1007/s11064-018-2548-1. Epub 2018 May 17. Neurochem Res. 2018. Retraction in: Neurochem Res. 2025 Mar 26;50(2):127. doi: 10.1007/s11064-025-04382-2. PMID: 29774448 Retracted.
-
Neuronal migration disorders: Focus on the cytoskeleton and epilepsy.Neurobiol Dis. 2016 Aug;92(Pt A):18-45. doi: 10.1016/j.nbd.2015.08.003. Epub 2015 Aug 20. Neurobiol Dis. 2016. PMID: 26299390 Free PMC article. Review.
-
Zebrafish Rohon-Beard neuron development: cdk5 in the midst.Neurochem Res. 2009 Jun;34(6):1129-37. doi: 10.1007/s11064-008-9885-4. Epub 2008 Dec 9. Neurochem Res. 2009. PMID: 19067160 Free PMC article. Review.
-
Pharmacogenetic analysis reveals a post-developmental role for Rac GTPases in Caenorhabditis elegans GABAergic neurotransmission.Genetics. 2009 Dec;183(4):1357-72. doi: 10.1534/genetics.109.106880. Epub 2009 Sep 21. Genetics. 2009. PMID: 19797046 Free PMC article.
-
Ectopic granule cells of the rat dentate gyrus.Dev Neurosci. 2007;29(1-2):14-27. doi: 10.1159/000096208. Dev Neurosci. 2007. PMID: 17148946 Free PMC article. Review.
References
-
- Allen KM, Walsh CA (1999) Genes that regulate neuronal migration in the cerebral cortex. Epilepsy Res 36: 143-154. - PubMed
-
- Austin JE, Buckmaster PS (2004) Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol 476: 205-218. - PubMed
-
- Buckmaster PS, Dudek FE (1997) Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol 77: 2685-2696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
