Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro
- PMID: 15483131
- PMCID: PMC6730063
- DOI: 10.1523/JNEUROSCI.2113-04.2004
Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro
Abstract
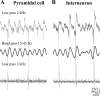
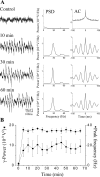
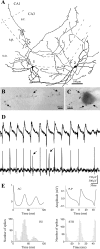
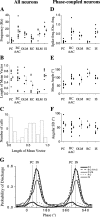
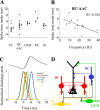
Gamma frequency (30-100 Hz) network oscillations occur in the intact hippocampus during awake, attentive behavior. Here, we explored the underlying cellular mechanisms in an in vitro model of persistent gamma-frequency oscillations, induced by bath application of 20 microm carbachol in submerged hippocampal slices at 30 +/- 1 degrees C. Current-source density analysis of the field oscillation revealed a prominent alternating sink-source pair in the perisomatic and apical dendritic regions of CA3. To elucidate the active events generating these extracellular dipoles, we examined the firing properties of distinct neuron types. Visually guided unit recordings were obtained from individual CA3 neurons followed by intracellular labeling for anatomical identification. Pyramidal cells fired at 2.82 +/- 0.7 Hz, close to the negative peak of the oscillation (0.03 +/- 0.65 msec), and often in conjunction with a negative spike-like component of the field potential. In contrast, all phase-coupled interneurons fired after this negative peak. Perisomatic inhibitory interneurons fired at high frequency (18.1 +/- 2.7 Hz), shortly after the negative peak (1.97 +/- 0.95 msec) and were strongly phase-coupled. Dendritic inhibitory interneurons fired at lower frequency (8.4 +/- 2.4 Hz) and with less fidelity and a longer delay after the negative peak (4.3 +/- 1.1 msec), whereas interneurons with cell body in the stratum radiatum often showed no phase relationship with the field oscillation. The phase and spike time data of individual neurons, together with the current-source density analysis, support a synaptic feedback model of gamma oscillations primarily involving pyramidal cells and inhibitory cells targeting their perisomatic region.
Figures



References
-
- Acsády L, Gorcs TJ, Freund TF (1996) Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience 73: 317-334. - PubMed
-
- Boddeke HW, Best R, Boeijinga PH (1997) Synchronous 20 Hz rhythmic activity in hippocampal networks induced by activation of metabotropic glutamate receptors in vitro. Neuroscience 76: 653-658. - PubMed
-
- Buzsáki G, Chrobak JJ (1995) Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol 5: 504-510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
