Latrophilin fragments behave as independent proteins that associate and signal on binding of LTX(N4C)
- PMID: 15483624
- PMCID: PMC526461
- DOI: 10.1038/sj.emboj.7600443
Latrophilin fragments behave as independent proteins that associate and signal on binding of LTX(N4C)
Abstract
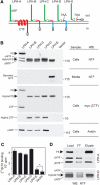
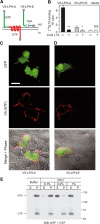
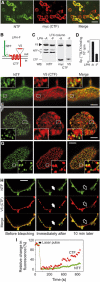
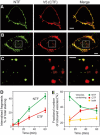
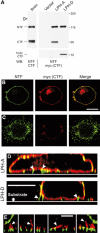
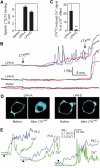
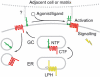
Heptahelical, or G-protein-coupled, receptors control many cellular functions and normally consist of one polypeptide chain. In contrast, heptahelical receptors that belong to the long N-terminus, group B (LNB) family are cleaved constitutively into two fragments. The N-terminal fragments (NTFs) resemble cell-adhesion proteins and the C-terminal fragments (CTFs) are typical G-protein-coupled receptors (GPCRs) with seven transmembrane regions. However, the functional roles of this cleavage and of any subsequent NTF-CTF interactions remain to be identified. Using latrophilin, a well-studied member of the LNB family, we now demonstrate that cleavage is critical for delivery of this receptor to the cell surface. On the plasma membrane, NTF and CTF behave as separate membrane proteins involved, respectively, in cell-surface reception and signalling. The two fragments can also internalise independently. However, separated NTF and CTF can re-associate on solubilisation. Agonist binding to NTF on the cell surface also induces re-association of fragments and provokes signal transduction via CTF. These findings define a novel principle of structural and functional organisation of the cleaved, two-subunit GPCRs.
Figures


References
-
- Abe J, Fukuzawa T, Hirose S (2002) Cleavage of Ig-Hepta at a ‘SEA' module and at a conserved G protein-coupled receptor proteolytic site. J Biol Chem 277: 23391–23398 - PubMed
-
- Ashton AC, Volynski KE, Lelianova VG, Orlova EV, Van Renterghem C, Canepari M, Seagar M, Ushkaryov YA (2001) Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J Biol Chem 276: 44695–44703 - PubMed
-
- Bouvier M (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci 2: 274–286 - PubMed
-
- Chang GW, Stacey M, Kwakkenbos MJ, Hamann J, Gordon S, Lin HH (2003) Proteolytic cleavage of the EMR2 receptor requires both the extracellular stalk and the GPS motif. FEBS Lett 547: 145–150 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

