DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains
- PMID: 15485915
- PMCID: PMC522253
- DOI: 10.1128/MCB.24.21.9478-9486.2004
DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains
Abstract
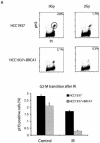
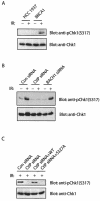
The BRCA1 C-terminal (BRCT) domain has recently been implicated as a phospho-protein binding domain. We demonstrate here that a CTBP-interacting protein CtIP interacts with BRCA1 BRCT domains in a phosphorylation-dependent manner. The CtIP/BRCA1 complex only exists in G(2) phase and is required for DNA damage-induced Chk1 phosphorylation and the G(2)/M transition checkpoint. However, the CtIP/BRCA1 complex is not required for the damage-induced G(2) accumulation checkpoint, which is controlled by a separate BRCA1/BACH1 complex. Taken together, these data not only implicate CtIP as a critical player in cell cycle checkpoint control but also provide molecular mechanisms by which BRCA1 controls multiple cell cycle transitions after DNA damage.
Figures







References
-
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
-
- Cantor, S. B., D. W. Bell, S. Ganesan, E. M. Kass, R. Drapkin, S. Grossman, D. C. Wahrer, D. C. Sgroi, W. S. Lane, D. A. Haber, and D. M. Livingston. 2001. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105:149-160. - PubMed
-
- Chen, J. 2000. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 60:5037-5039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
