Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105
- PMID: 15485931
- PMCID: PMC522219
- DOI: 10.1128/MCB.24.21.9658-9667.2004
Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105
Abstract
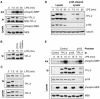
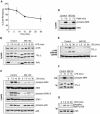
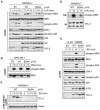
The MEK kinase TPL-2 (also known as Cot) is required for lipopolysaccharide (LPS) activation of the extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase cascade in macrophages and consequent upregulation of genes involved in innate immune responses. In resting cells, TPL-2 forms a stoichiometric complex with NF-kappaB1 p105, which negatively regulates its MEK kinase activity. Here, it is shown that lipopolysaccharide (LPS) stimulation of primary macrophages causes the release of both long and short forms of TPL-2 from p105 and that TPL-2 MEK kinase activity is restricted to this p105-free pool. Activation of TPL-2, MEK, and ERK by LPS is also demonstrated to require proteasome-mediated proteolysis. p105 is known to be proteolysed by the proteasome following stimulus-induced phosphorylation of two serines in its PEST region by the IkappaB kinase (IKK) complex. Expression of a p105 point mutant, which is not susceptible to signal-induced proteolysis, in RAW264.7 macrophages impairs LPS-induced release of TPL-2 from p105 and its subsequent activation of MEK. Furthermore, expression of wild-type but not mutant p105 reconstitutes LPS stimulation of MEK and ERK phosphorylation in primary NF-kappaB1-deficient macrophages. Consistently, pharmacological blockade of IKK inhibits LPS-induced release of TPL-2 from p105 and TPL-2 activation. These data show that IKK-induced p105 proteolysis is essential for LPS activation of TPL-2, thus revealing a novel function of IKK in the regulation of the ERK MAP kinase cascade.
Figures


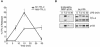
References
-
- Aoki, M., F. Hamada, T. Sugimoto, S. Sumida, T. Akiyama, and K. Toyoshima. 1993. The human cot proto-oncogene encodes two protein serine/threonine kinases with different transforming activities by alternative initiation of translation. J. Biol. Chem. 268:22723-22732. - PubMed
-
- Beinke, S., M. P. Belich, and S. C. Ley. 2002. The death domain of NF-κB1 p105 is essential for signal-induced p105 proteolysis. J. Biol. Chem. 277:24162-24168. - PubMed
-
- Belich, M. P., A. Salmeron, L. H. Johnston, and S. C. Ley. 1999. TPL-2 kinase regulates the proteolysis of the NF-κB inhibitory protein NF-κB1 p105. Nature 397:363-368. - PubMed
-
- Burke, J. R., M. A. Pattoli, K. R. Gregor, P. J. Brassil, J. F. MacMaster, K. W. McIntyre, X. Yang, V. S. Iotzova, W. Clarke, J. Strnad, Y. Qiu, and F. C. Zusi. 2003. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J. Biol. Chem. 278:1450-1456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
