Fully human IgG and IgM antibodies directed against the carcinoembryonic antigen (CEA) Gold 4 epitope and designed for radioimmunotherapy (RIT) of colorectal cancers
- PMID: 15488142
- PMCID: PMC526287
- DOI: 10.1186/1471-2407-4-75
Fully human IgG and IgM antibodies directed against the carcinoembryonic antigen (CEA) Gold 4 epitope and designed for radioimmunotherapy (RIT) of colorectal cancers
Abstract
Background: Human monoclonal antibodies (MAbs) are needed for colon cancer radioimmunotherapy (RIT) to allow for repeated injections. Carcinoembryonic antigen (CEA) being the reference antigen for immunotargeting of these tumors, we developed human anti-CEA MAbs.
Methods: XenoMouse-G2 animals were immunized with CEA. Among all the antibodies produced, two of them, VG-IgG2kappa and VG-IgM, were selected for characterization in vitro in comparison with the human-mouse chimeric anti-CEA MAb X4 using flow cytometry, surface plasmon resonance, and binding to radiolabeled soluble CEA and in vivo in human colon carcinoma LS174T bearing nude mice.
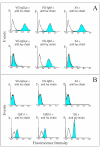
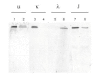
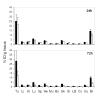
Results: Flow cytometry analysis demonstrated binding of MAbs on CEA-expressing cells without any binding on NCA-expressing human granulocytes. In a competitive binding assay using five reference MAbs, directed against the five Gold CEA epitopes, VG-IgG2kappa and VG-IgM were shown to be directed against the Gold 4 epitope. The affinities of purified VG-IgG2kappa and VG-IgM were determined to be 0.19 +/- 0.06 x 10(8) M(-1) and 1.30 +/- 0.06 x 10(8) M(-1), respectively, as compared with 0.61 +/- 0.05 x 10(8) M(-1) for the reference MAb X4. In a soluble phase assay, the binding capacities of VG-IgG2kappa and VG-IgM to soluble CEA were clearly lower than that of the control chimeric MAb X4. A human MAb concentration of about 10(-7) M was needed to precipitate approximatively 1 ng 125I-rhCEA as compared with 10(-9) M for MAb X4, suggesting a preferential binding of the human MAbs to solid phase CEA. In vivo, 24 h post-injection, 125I-VG-IgG2kappa demonstrated a high tumor uptake (25.4 +/- 7.3%ID/g), close to that of 131I-X4 (21.7 +/- 7.2%ID/g). At 72 h post-injection, 125I-VG-IgG2kappa was still concentrated in the tumor (28.4 +/- 11.0%ID/g) whereas the tumor concentration of 131I-X4 was significantly reduced (12.5 +/- 4.8%ID/g). At no time after injection was there any accumulation of the radiolabeled MAbs in normal tissues. A pertinent analysis of VG-IgM biodistribution was not possible in this mouse model in which IgM displays a very short half-life due to poly-Ig receptor expression in the liver.
Conclusion: Our human anti-CEA IgG2kappa is a promising candidate for radioimmunotherapy in intact form, as F(ab')2 fragments, or as a bispecific antibody.
Figures

Similar articles
-
Biokinetics of a F(ab')3 iodine-131 labeled antigen binding construct (Mab 35) directed against CEA in patients with colorectal carcinoma.Cancer Biother Radiopharm. 2001 Oct;16(5):371-9. doi: 10.1089/108497801753354276. Cancer Biother Radiopharm. 2001. PMID: 11776754
-
Swine monoclonal antibodies of high affinity and specificity to carcinoembryonic antigen.J Natl Cancer Inst. 1987 Aug;79(2):337-42. J Natl Cancer Inst. 1987. PMID: 2439734
-
Dosimetric evaluation and radioimmunotherapy of anti-tumour multivalent Fab' fragments.Br J Cancer. 1999 Nov;81(6):972-80. doi: 10.1038/sj.bjc.6690795. Br J Cancer. 1999. PMID: 10576653 Free PMC article.
-
Radioimmunotherapy of colorectal cancer liver metastases: combination with radiotherapy.Ann N Y Acad Sci. 2000 Jun;910:263-9; discussion 269-70. doi: 10.1111/j.1749-6632.2000.tb06714.x. Ann N Y Acad Sci. 2000. PMID: 10911919 Review.
-
Radioiodinated anti-CEA monoclonal antibody NP-4 F(ab´)2 fragment.2006 Mar 23 [updated 2008 Apr 10]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2006 Mar 23 [updated 2008 Apr 10]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641898 Free Books & Documents. Review.
Cited by
-
In Vitro Characterization of 177Lu-DOTA-M5A Anti-Carcinoembryonic Antigen Humanized Antibody and HSP90 Inhibition for Potentiated Radioimmunotherapy of Colorectal Cancer.Front Oncol. 2022 Mar 31;12:849338. doi: 10.3389/fonc.2022.849338. eCollection 2022. Front Oncol. 2022. PMID: 35433442 Free PMC article.
-
Human monoclonal antibodies from transgenic mice.Handb Exp Pharmacol. 2008;181(181):69-97. doi: 10.1007/978-3-540-73259-4_4. Handb Exp Pharmacol. 2008. PMID: 18071942 Free PMC article. Review.
-
Adjuvant radioimmunotherapy trial with iodine-131-labeled anti-carcinoembryonic antigen monoclonal antibody F6 F(ab')2 after resection of liver metastases from colorectal cancer.Clin Cancer Res. 2008 Jun 1;14(11):3487-93. doi: 10.1158/1078-0432.CCR-07-4698. Clin Cancer Res. 2008. PMID: 18519781 Free PMC article. Clinical Trial.
-
Recombinant chimeric antibody hCAb as a novel anti-human colorectal carcinoma agent.Mol Med. 2006 Sep-Oct;12(9-10):229-36. doi: 10.2119/2006-00021.Xiong. Mol Med. 2006. PMID: 17225871 Free PMC article.
References
-
- Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, Lister TA, Stagg RJ, Tidmarsh GF, Kroll S, Wahl RL, Knox SJ, Vose JM. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918–3928. - PubMed
-
- Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-Lopez AJ, Multani P, White CA. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. - DOI - PubMed
-
- Sharkey RM, Brenner A, Burton J, Hajjar G, Toder SP, Alavi A, Matthies A, Tsai DE, Schuster SJ, Stadtmauer EA, Czuczman MS, Lamonica D, Kraeber-Bodere F, Mahe B, Chatal JF, Rogatko A, Mardirrosian G, Goldenberg DM. Radioimmunotherapy of non-Hodgkin's lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): do tumor targeting and dosimetry predict therapeutic response? J Nucl Med. 2003;44:2000–2018. - PubMed
-
- Mach JP, Carrel S, Forni M, Ritschard J, Donath A, Alberto P. Tumor localization of radiolabeled antibodies against carcinoembryonic antigen in patients with carcinoma: a critical evaluation. N Engl J Med. 1980;303:5–10. - PubMed
-
- Goldenberg DM, DeLand F, Kim E, Bennett S, Primus FJ, VanNagell JR, Estes N, DeSimone P, Rayburn P. Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N Engl J Med. 1978;298:1384–1388. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

