Ceramide, a target for antiretroviral therapy
- PMID: 15489273
- PMCID: PMC524434
- DOI: 10.1073/pnas.0402874101
Ceramide, a target for antiretroviral therapy
Abstract
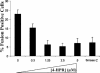
Studies of ceramide metabolism and function in a wide range of biological processes have revealed a role for this lipid in regulating key cellular responses. Our research on the role of sphingolipids in HIV entry has led to the hypothesis that modulation of ceramide levels in target cells affects their susceptibility to HIV infection by rearranging HIV receptors. Cellular ceramide levels were modulated by application of pharmacological agents such as N-(4-hydroxyphenyl)retinamide (4-HPR, fenretinide), by treatment with sphingomyelinase (Smase), or by exogenous addition of long-chain ceramide, and determined after metabolic incorporation of [3H]sphingosine. Infectivity assays were performed by using a HeLa-derived indicator cell line, TZM-bl, CD4+ lymphocytes, and monocytes. We observed a dose-dependent inhibition by 4-HPR of infection of TZM-bl cells by a broad range of HIV-1 isolates at low micromolar concentrations with an IC50 of <1 microM for most isolates tested. Nearly complete inhibition was seen at 5 microM, a dose that enhanced ceramide levels by 50-100%, yet was nontoxic to the cells. Treating cells with other pharmacological agents that enhanced ceramide levels, with Smase, or exogenous addition of long-chain ceramide also resulted in inhibition of HIV-1 infection. Enhancing ceramide levels in CD4+ lymphocytes and in monocyte-derived macrophages with 4-HPR or Smase significantly reduced infectivity without toxicity. The minimal toxicity of normal cells exposed to 4-HPR should make the drug exceedingly suitable as an anti-HIV therapeutic.
Figures

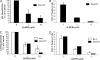
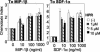
References
-
- Gallo, S. A., Finnegan, C. M., Viard, M., Raviv, Y., Dimitrov, A., Rawat, S. S., Puri, A., Durell, S. & Blumenthal, R. (2003) Biochim. Biophys. Acta 1614, 36-50. - PubMed
-
- Rawat, S. S., Viard, M., Gallo, S. A., Rein, A., Blumenthal, R. & Puri, A. (2003) Mol. Membr. Biol. 20, 243-254. - PubMed
-
- Hannun, Y. A. & Obeid, L. M. (2002) J. Biol. Chem. 277, 25847-25850. - PubMed
-
- Cremesti, A. E., Goni, F. M. & Kolesnick, R. (2002) FEBS Lett. 531, 47-53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

