The active spatial organization of the beta-globin locus requires the transcription factor EKLF
- PMID: 15489291
- PMCID: PMC529536
- DOI: 10.1101/gad.317004
The active spatial organization of the beta-globin locus requires the transcription factor EKLF
Abstract
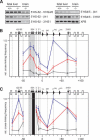
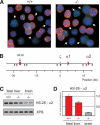
Three-dimensional organization of a gene locus is important for its regulation, as recently demonstrated for the beta-globin locus. When actively expressed, the cis-regulatory elements of the beta-globin locus are in proximity in the nuclear space, forming a compartment termed the Active Chromatin Hub (ACH). However, it is unknown which proteins are involved in ACH formation. Here, we show that EKLF, an erythroid transcription factor required for adult beta-globin gene transcription, is also required for ACH formation. We conclude that transcription factors can play an essential role in the three-dimensional organization of gene loci.
Figures



References
-
- Armstrong J.A., Bieker, J.J., and Emerson, B.M. 1998. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell 95: 93-104. - PubMed
-
- Bender M.A., Bulger, M., Close, J., and Groudine, M. 2000. β-globin gene switching and DNase I sensitivity of the endogenous β-globin locus in mice do not require the locus control region. Mol. Cell 5: 387-393. - PubMed
-
- Bieker J.J. 2001. Kruppel-like factors: Three fingers in many pies. J. Biol. Chem. 276: 34355-34358. - PubMed
-
- Carter D., Chakalova, L., Osborne, C.S., Dai, Y.F., and Fraser, P. 2002. Long-range chromatin regulatory interactions in vivo. Nat. Genet. 32: 623-626. - PubMed
-
- De Laat W. and Grosveld, F. 2003. Spatial organization of gene expression: The Active Chromatin Hub. Chromosome Res. 5: 447-459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
