Elucidation and structural analysis of conserved pools for genome-scale metabolic reconstructions
- PMID: 15489308
- PMCID: PMC1305013
- DOI: 10.1529/biophysj.104.043489
Elucidation and structural analysis of conserved pools for genome-scale metabolic reconstructions
Abstract
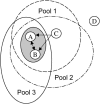
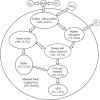
In this article, we introduce metabolite concentration coupling analysis (MCCA) to study conservation relationships for metabolite concentrations in genome-scale metabolic networks. The analysis allows the global identification of subsets of metabolites whose concentrations are always coupled within common conserved pools. Also, the minimal conserved pool identification (MCPI) procedure is developed for elucidating conserved pools for targeted metabolites without computing the entire basis conservation relationships. The approaches are demonstrated on genome-scale metabolic reconstructions of Helicobacter pylori, Escherichia coli, and Saccharomyces cerevisiae. Despite significant differences in the size and complexity of the examined organism's models, we find that the concentrations of nearly all metabolites are coupled within a relatively small number of subsets. These correspond to the overall exchange of carbon molecules into and out of the networks, interconversion of energy and redox cofactors, and the transfer of nitrogen, sulfur, phosphate, coenzyme A, and acyl carrier protein moieties among metabolites. The presence of large conserved pools can be viewed as global biophysical barriers protecting cellular systems from stresses, maintaining coordinated interconversions between key metabolites, and providing an additional mode of global metabolic regulation. The developed approaches thus provide novel and versatile tools for elucidating coupling relationships between metabolite concentrations with implications in biotechnological and medical applications.
Figures






References
-
- Almaas, L., B. Kovacs, T. Vicsek, Z. N. Oltval, and A.-L. Barabasi. 2004. Global organization of metabolic fluxes in the bacterium Escherichia coli. Nature. 427:839–843. - PubMed
-
- Atkinson, D. E. 1968. The energy charge of the adenylate pool as a regulatory parameter. Interactions with feedback modifiers. Biochemistry. 7:4030–4034. - PubMed
-
- Bakker, B. M., P. A. M. Michels, F. R. Opperdoes, and H. V. Westerhoff. 1999. What controls glycolysis in bloodstream form Trypanosoma brucei? J. Biol. Chem. 274:14551–14559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

