Control of glycolytic oscillations by temperature
- PMID: 15489309
- PMCID: PMC1305041
- DOI: 10.1529/biophysj.104.043398
Control of glycolytic oscillations by temperature
Abstract
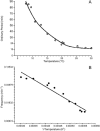
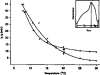
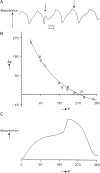
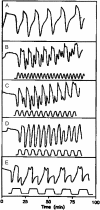
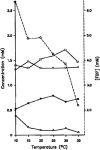
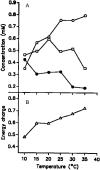
External control of oscillatory glycolysis in yeast extract has been performed by application of either homogeneous temperature oscillations or stationary, spatial temperature gradients. Entrainment of the glycolytic oscillations by the 1/2- and 1/3-harmonic, as well as the fundamental input frequency, could be observed. From the phase response curve to a single temperature pulse, a distinct sensitivity of NADH-oxidizing processes, compared with NAD-reducing processes, is visible. Determination of glycolytic intermediates shows that the feedback-regulated phosphofructokinase as well as the glyceraldehyde-3-phosphate dehydrogenase are the most temperature-sensitive steps of glycolysis. We also find strong concentration changes in ATP and AMP at varying temperatures and, accordingly, in the energy charge. Construction of a feedback loop for spatial control of temperature by means of a Peltier element allowed us to apply a temperature gradient to the yeast extract. With this setup it is possible to initiate traveling waves and to control the wave velocity.
Figures
References
-
- Alcantara, F., and M. Monk. 1974. Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J. Gen. Microbiol. 85:321–334. - PubMed
-
- Bergmeyer, H. U. 1974. Methods of Enzymatic Analysis, Vol. III. Verlag Chemie Weinheim/Academic Press, New York, NY and London, UK.
-
- Berridge, M. J., and P. E. Rapp. 1979. A comparative survey of the function, mechanism and control of cellular oscillations. J. Exp. Biol. 81:217–279. - PubMed
-
- Betz, A., and B. Chance. 1965. Influence of inhibitors and temperature on the oscillation of reduced pyridine nucleotides in yeast cells. Arch. Biochem. Biophys. 109:579–584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

