Coccidioidomycosis: host response and vaccine development
- PMID: 15489350
- PMCID: PMC523560
- DOI: 10.1128/CMR.17.4.804-839.2004
Coccidioidomycosis: host response and vaccine development
Abstract
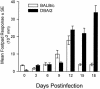
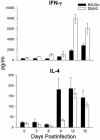
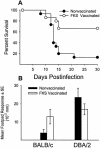
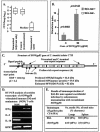
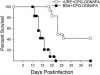
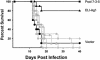
Coccidioidomycosis is caused by the dimorphic fungi in the genus Coccidioides. These fungi live as mycelia in the soil of desert areas of the American Southwest, and when the infectious spores, the arthroconidia, are inhaled, they convert into the parasitic spherule/endospore phase. Most infections are mild, but these organisms are frank pathogens and can cause severe lethal disease in fully immunocompetent individuals. While there is increased risk of disseminated disease in certain racial groups and immunocompromised persons, the fact that there are hosts who contain the initial infection and exhibit long-term immunity to reinfection supports the hypothesis that a vaccine against these pathogens is feasible. Multiple studies have shown that protective immunity against primary disease is associated with T-helper 1 (Th-1)-associated immune responses. The single best vaccine in animal models, formalin-killed spherules (FKS), was tested in a human trial but was not found to be significantly protective. This result has prompted studies to better define immunodominant Coccidioides antigen with the thought that a subunit vaccine would be protective. These efforts have defined multiple candidates, but the single best individual immunogen is the protein termed antigen 2/proline-rich antigen (Ag2/PRA). Studies in multiple laboratories have shown that Ag2/PRA as both protein and genetic vaccines provides significant protection against mice challenged systemically with Coccidioides. Unfortunately, compared to the FKS vaccine, it is significantly less protective as measured by both assays of reduction in fungal CFU and assays of survival. The capacity of Ag2/PRA to induce only partial protection was emphasized when animals were challenged intranasally. Thus, there is a need to define new candidates to create a multivalent vaccine to increase the effectiveness of Ag2/PRA. Efforts of genomic screening using expression library immunization or bioinformatic approaches to identify new candidates have revealed at least two new protective proteins, expression library immunization antigen 1 (ELI-Ag1) and a beta-1,3-glucanosyltransferase (GEL-1). In addition, previously discovered antigens such as Coccidioides-specific antigen (CSA) should be evaluated in assays of protection. While studies have yet to be completed with combinations of the current candidates, the hypothesis is that with increased numbers of candidates in a multivalent vaccine, there will be increased protection. As the genome sequences of the two Coccidioides strains which are under way are completed and annotated, the effort to find new candidates can increase to provide a complete genomic scan for immunodominant proteins. Thus, much progress has been made in the discovery of subunit vaccine candidates against Coccidioides and there are several candidates showing modest levels of protection, but for complete protection against pulmonary challenge we need to continue the search for additional candidates.
Figures









References
-
- Abuodeh, R. O., L. F. Shubitz, E. Siegel, S. Snyder, T. Peng, K. I. Orsborn, E. Brummer, D. A. Stevens, and J. N. Galgiani. 1999. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect. Immun. 67:2935-2940. - PMC - PubMed
-
- Albert, E. D., M. R. Mickey, and P. I. Terasaki. 1973. Genetics of the HLA system in four populations: American-Caucasians, Japanese-Americans, American Negroes, and Mexican Americans, p. 233-240. In J. Dausett and J. Colombani (ed.), Histocompatibility testing. The Williams & Wilkins Co., Baltimore, Md.
-
- Ampel, N. M. 1994. In vitro production of tumor necrosis factor-alpha by adherent human peripheral blood mononuclear cells incubated with killed coccidioidal arthroconidia and spherules. Cell. Immunol. 153:248-255. - PubMed
-
- Ampel, N. M., and L. Christian. 2000. Flow cytometric assessment of human peripheral blood mononuclear cells in response to a coccidioidal antigen. Med. Mycol. 38:127-132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

