Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy
- PMID: 15489359
- PMCID: PMC523563
- DOI: 10.1128/CMR.17.4.1012-1030.2004
Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy
Abstract
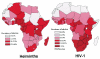
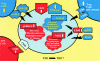
Chronic immune activation is one of the hallmarks of human immunodeficiency virus (HIV) infection. It is present also, with very similar characteristics, in very large human populations infested with helminthic infections. We have tried to review the studies addressing the changes in the immune profiles and responses of hosts infected with either one of these two chronic infections. Not surprisingly, several of the immune derangements and impairments seen in HIV infection, and considered by many to be the "specific" effects of HIV, can be found in helminth-infected but HIV-noninfected individuals and can thus be accounted for by the chronic immune activation itself. A less appreciated element in chronic immune activation is the immune suppression and anergy which it may generate. Both HIV and helminth infections represent this aspect in a very wide and illustrative way. Different degrees of anergy and immune hyporesponsiveness are present in these infections and probably have far-reaching effects on the ability of the host to cope with these and other infections. Furthermore, they may have important practical implications, especially with regard to protective vaccinations against AIDS, for populations chronically infected with helminths and therefore widely anergic. The current knowledge of the mechanisms responsible for the generation of anergy by chronic immune activation is thoroughly reviewed.
Figures



References
-
- Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. - PubMed
-
- Adams, J. F., E. H. Scholvinck, R. P. Gie, P. C. Potter, N. Beyers, and A. D. Beyers. 1999. Decline in total serum IgE after treatment for tuberculosis. Lancet 353:2030-2033. - PubMed
-
- Alberola-Ila, J., and G. Hernandez-Hoyos. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191:79-96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

