Enhanced low vision rehabilitation for people with age related macular degeneration: a randomised controlled trial
- PMID: 15489491
- PMCID: PMC1772403
- DOI: 10.1136/bjo.2003.037457
Enhanced low vision rehabilitation for people with age related macular degeneration: a randomised controlled trial
Abstract
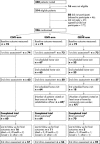
Aim: To compare the effectiveness of three models of low vision rehabilitation for people with age related macular degeneration (AMD) referred for low vision rehabilitation (LVR): (a) an enhanced low vision rehabilitation model (ELVR) including supplementary home based low vision rehabilitation; (b) conventional low vision rehabilitation (CLVR) based in a hospital clinic; (c) CLVR with home visits that did not include rehabilitation (CELVR), intended to act as a control for the additional contact time with ELVR.
Method: A single centre parallel group randomised controlled trial in participants' homes and the low vision clinic, Manchester Royal Eye Hospital. People referred for LVR with a primary diagnosis of AMD and visual acuity worse than 6/18 in both eyes and equal to or better than 1/60 in the better eye. The main outcome measures were vision specific quality of life (QoL) (primary outcome, VCM1) and generic health related QoL (SF-36); psychological adjustment to vision loss; measured task performance; restriction in everyday activities; use of low vision aids (LVAs).
Results: 226 participants were recruited (median age 82 years); 194 completed the trial (86%). Except for SF-36 physical and mental component summary scores, arms did not differ significantly for any of the outcomes. Differences for the VCM1 were ELVR v CLVR, 0.06 (95% CI to 0.17 to 0.30, p = 0.60); ELVR v CELVR, 0.12 (95% CI to 0.11 to 0.34, p = 0.31); CELVR v CLVR, -0.05 (95% CI -0.29 to 0.18, p = 0.64). Differences for the SF-36 favoured CLVR compared to ELVR (ELVR v CLVR: physical = -6.05, 95% CI -10.2 to -1.91, p = 0.004; mental = -4.04, 95% CI -7.44 to -0.65, p = 0.02). At 12 months, 94% of participants reported using at least one LVA.
Conclusion: ELVR was no more effective than CLVR. Researchers should be wary of proposing new LVR interventions without preliminary evidence of effectiveness, given the manifest lack of effectiveness of the model of enhanced LVR evaluated in the trial.
Figures
References
-
- Harvey PT. Common eye diseases of elderly people: identifying and treating causes of vision loss. Gerontology 2003;49:1–11. - PubMed
-
- Rubin GS, Bandee-Roche K, Huang GH, et al. The association of multiple visual impairments with self-reported visual disability: SEE project. Invest Ophthalmol Vis Sci 2001;42:64–72. - PubMed
-
- Williams RA, Brody BL, Thomas RG, et al. The psychosocial impact of macular degeneration. Arch Ophthalmol 1998;116:514–20. - PubMed
-
- Mangione CM, Gutierrez PR, Lowe G, et al. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol 1999;128:45–53. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
