Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice
- PMID: 15489961
- PMCID: PMC522244
- DOI: 10.1172/JCI21633
Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice
Abstract
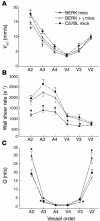
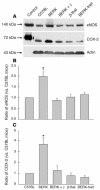
In sickle cell disease, intravascular sickling and attendant flow abnormalities underlie the chronic inflammation and vascular endothelial abnormalities. However, the relationship between sickling and vascular tone is not well understood. We hypothesized that sickling-induced vaso-occlusive events and attendant oxidative stress will affect microvascular regulatory mechanisms. In the present studies, we have examined whether microvascular abnormalities expressed in sickle transgenic-knockout Berkeley (BERK) mice (which express exclusively human alpha- and beta(S)-globins with <1% gamma-globin levels) are amenable to correction with increased levels of antisickling fetal hemoglobin (HbF). In BERK mice, sickling, increased oxidative stress, and hemolytic anemia are accompanied by vasodilation, compensatory increases in eNOS and COX-2, and attenuated vascular responses to NO-mediated vasoactive stimuli and norepinephrine. The hypotension and vasodilation (required for adequate oxygen delivery in the face of chronic anemia) are mediated by non-NO vasodilators (i.e., prostacyclin) as evidenced by induction of COX-2. In BERK mice, the resistance to NO-mediated vasodilators is associated with increased oxidative stress and hemolytic rate, and in BERK + gamma mice (expressing 20% HbF), an improved response to these stimuli is associated with reduced oxidative stress and hemolytic rate. Furthermore, BERK + gamma mice show normalization of vessel diameters, and eNOS and COX-2 expression. These results demonstrate a strong relationship between sickling and microvascular function in sickle cell disease.
Figures





References
-
- Kaul DK, Fabry ME, Nagel RL. The pathophysiology of vascular obstruction in the sickle syndromes. Blood Rev. 1996;10:29–44. - PubMed
-
- Osarogiagbon UR, et al. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. - PubMed
-
- Oh SO, Ibe BO, Johnson C, Kurantsin-Mills J, Raj JU. Platelet-activating factor in plasma of patients with sickle cell disease in steady state. J. Lab. Clin. Med. 1997;130:191–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

