The forces that position a mitotic spindle asymmetrically are tethered until after the time of spindle assembly
- PMID: 15492042
- PMCID: PMC2172534
- DOI: 10.1083/jcb.200406008
The forces that position a mitotic spindle asymmetrically are tethered until after the time of spindle assembly
Abstract
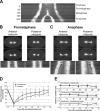
Regulation of the mitotic spindle's position is important for cells to divide asymmetrically. Here, we use Caenorhabditis elegans embryos to provide the first analysis of the temporal regulation of forces that asymmetrically position a mitotic spindle. We find that asymmetric pulling forces, regulated by cortical PAR proteins, begin to act as early as prophase and prometaphase, even before the spindle forms and shifts to a posterior position. The spindle does not shift asymmetrically during these early phases due to a tethering force, mediated by astral microtubules that reach the anterior cell cortex. We show that this tether is normally released after spindle assembly and independently of anaphase entry. Monitoring microtubule dynamics by photobleaching segments of microtubules during anaphase revealed that spindle microtubules do not undergo significant poleward flux in C. elegans. Together with the known absence of anaphase A, these data suggest that the major forces contributing to chromosome separation during anaphase originate outside the spindle. We propose that the forces positioning the mitotic spindle asymmetrically are tethered until after the time of spindle assembly and that these same forces are used later to drive chromosome segregation at anaphase.
Figures





Similar articles
-
Microtubule Feedback and LET-99-Dependent Control of Pulling Forces Ensure Robust Spindle Position.Biophys J. 2018 Dec 4;115(11):2189-2205. doi: 10.1016/j.bpj.2018.10.010. Epub 2018 Oct 19. Biophys J. 2018. PMID: 30447992 Free PMC article.
-
Visualization of dynein-dependent microtubule gliding at the cell cortex: implications for spindle positioning.J Cell Biol. 2011 Aug 8;194(3):377-86. doi: 10.1083/jcb.201103128. J Cell Biol. 2011. PMID: 21825072 Free PMC article.
-
Physical Limits on the Precision of Mitotic Spindle Positioning by Microtubule Pushing forces: Mechanics of mitotic spindle positioning.Bioessays. 2017 Nov;39(11):10.1002/bies.201700122. doi: 10.1002/bies.201700122. Epub 2017 Sep 28. Bioessays. 2017. PMID: 28960439 Free PMC article. Review.
-
The coordination of spindle-positioning forces during the asymmetric division of the Caenorhabditis elegans zygote.EMBO Rep. 2021 May 5;22(5):e50770. doi: 10.15252/embr.202050770. Epub 2021 Apr 26. EMBO Rep. 2021. PMID: 33900015 Free PMC article.
-
Spindle positioning during the asymmetric first cell division of Caenorhabditis elegans embryos.Novartis Found Symp. 2001;237:164-75; discussion 176-81. doi: 10.1002/0470846666.ch13. Novartis Found Symp. 2001. PMID: 11444042 Review.
Cited by
-
Cell cycle-regulated cortical dynein/dynactin promotes symmetric cell division by differential pole motion in anaphase.Mol Biol Cell. 2012 Sep;23(17):3380-90. doi: 10.1091/mbc.E12-02-0109. Epub 2012 Jul 18. Mol Biol Cell. 2012. PMID: 22809624 Free PMC article.
-
Stoichiometric interactions explain spindle dynamics and scaling across 100 million years of nematode evolution.Elife. 2020 Sep 23;9:e55877. doi: 10.7554/eLife.55877. Elife. 2020. PMID: 32966209 Free PMC article.
-
Functional genomic identification of genes required for male gonadal differentiation in Caenorhabditis elegans.Genetics. 2010 Jun;185(2):523-35. doi: 10.1534/genetics.110.116038. Epub 2010 Mar 22. Genetics. 2010. PMID: 20308279 Free PMC article.
-
Regulation of chromosome speeds in mitosis.Cell Mol Bioeng. 2013 Dec;6(4):418-430. doi: 10.1007/s12195-013-0297-4. Cell Mol Bioeng. 2013. PMID: 26405462 Free PMC article.
-
Evolutionary comparisons reveal a positional switch for spindle pole oscillations in Caenorhabditis embryos.J Cell Biol. 2013 May 27;201(5):653-62. doi: 10.1083/jcb.201210110. Epub 2013 May 20. J Cell Biol. 2013. PMID: 23690175 Free PMC article.
References
-
- Albertson, D.G. 1984. Formation of the first cleavage spindle in nematode embryos. Dev. Biol. 101:61–72. - PubMed
-
- Cleveland, D.W., Y. Mao, and K.F. Sullivan. 2003. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 112:407–421. - PubMed
-
- Colombo, K., S.W. Grill, R.J. Kimple, F.S. Willard, D.P. Siderovski, and P. Gönczy. 2003. Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science. 300:1957–1961. - PubMed

