A pentadecapeptide fragment of islet neogenesis-associated protein increases beta-cell mass and reverses diabetes in C57BL/6J mice
- PMID: 15492571
- PMCID: PMC1356495
- DOI: 10.1097/01.sla.0000143270.99191.10
A pentadecapeptide fragment of islet neogenesis-associated protein increases beta-cell mass and reverses diabetes in C57BL/6J mice
Abstract
Objective: The objective of this study was to demonstrate that islet neogenesis-associated protein (INGAP) peptide, a pentadecapeptide containing the biologically active portion of native INGAP, increases functional beta-cell mass in normal animals and can be used therapeutically to reverse hyperglycemia in streptozotocin-induced diabetes.
Summary background data: INGAP, a 175 amino acid pancreatic acinar cell protein, has been suggested to be implicated in beta-cell mass expansion.
Methods: In the first part of this study, normoglycemic hamsters were administered either 500 microg INGAP peptide (n = 30) or saline (n = 20) intraperitoneally daily and sacrificed after 10 or 30 days of treatment. Blood glucose and insulin levels were measured, and a histologic and morphometric analysis of the pancreas was performed to determine the effect of INGAP peptide on the endocrine pancreas. In the second part of the study, 6- to 8-week-old C57BL/6J mice (n = 8) were administered multiple low doses of the beta-cell toxin streptozotocin (STZ) inducing insulitis and hyperglycemia. The mice were then injected with INGAP peptide (n = 4) or saline (n = 4) for 39 days and sacrificed at 48 days. Two additional groups of diabetic mice were administered either a peptide composed of a scrambled sequence of amino acids from INGAP peptide (n = 5) or exendin-4 (n = 5), an incretin that has been associated with amelioration of hyperglycemia.
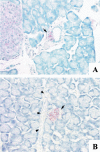
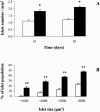
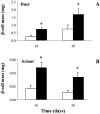
Results: Islet cell neogenesis was stimulated in INGAP-treated hamsters by 10 days. At 30 days, the foci of new endocrine cells had the appearance of mature islets. There was a 75% increase in islet number, with normal circulating levels of blood glucose and insulin. Administration of INGAP peptide to diabetic mice reversed the diabetic state in all animals, and this was associated with increased expression of PDX-1 in duct cells and islet cell neogenesis with a reduction of insulitis in the new islets. Diabetic mice treated with exendin-4 or a scrambled INGAP peptide did not revert from hyperglycemia.
Conclusion: Because there is a deficiency of beta-cell mass in both type-1 and type-2 diabetes, INGAP peptide stimulation of fully functional neoislet differentiation may provide a novel approach for diabetes therapy.
Figures

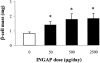



Similar articles
-
Islet Neogenesis Associated Protein (INGAP) induces the differentiation of an adult human pancreatic ductal cell line into insulin-expressing cells through stepwise activation of key transcription factors for embryonic beta cell development.Differentiation. 2015 Nov-Dec;90(4-5):77-90. doi: 10.1016/j.diff.2015.10.008. Epub 2015 Nov 11. Differentiation. 2015. PMID: 26558987
-
Possible relationship between changes in islet neogenesis and islet neogenesis-associated protein-positive cell mass induced by sucrose administration to normal hamsters.J Endocrinol. 2000 Jun;165(3):725-33. doi: 10.1677/joe.0.1650725. J Endocrinol. 2000. PMID: 10828857
-
Pancreatic duodenal homeobox-1 and islet neogenesis-associated protein: a possible combined marker of activateable pancreatic cell precursors.J Endocrinol. 2003 May;177(2):249-59. doi: 10.1677/joe.0.1770249. J Endocrinol. 2003. PMID: 12740013
-
The role of islet neogeneis-associated protein (INGAP) in pancreatic islet neogenesis.Curr Protein Pept Sci. 2009 Feb;10(1):37-45. doi: 10.2174/138920309787315211. Curr Protein Pept Sci. 2009. PMID: 19275671 Review.
-
The role of islet neogenesis-associated protein (INGAP) in islet neogenesis.Cell Biochem Biophys. 2007;48(2-3):127-37. doi: 10.1007/s12013-007-0028-3. Cell Biochem Biophys. 2007. PMID: 17709882 Review.
Cited by
-
Controversial Roles of Regenerating Family Proteins in Tissue Repair and Tumor Development.Biomedicines. 2024 Dec 26;13(1):24. doi: 10.3390/biomedicines13010024. Biomedicines. 2024. PMID: 39857608 Free PMC article. Review.
-
Antimicrobial protein REG3A regulates glucose homeostasis and insulin resistance in obese diabetic mice.Commun Biol. 2023 Mar 15;6(1):269. doi: 10.1038/s42003-023-04616-5. Commun Biol. 2023. PMID: 36918710 Free PMC article.
-
Regenerating proteins and their expression, regulation and signaling.Biomol Concepts. 2012 Feb;3(1):57-70. doi: 10.1515/bmc.2011.055. Epub 2011 Nov 10. Biomol Concepts. 2012. PMID: 22582090 Free PMC article.
-
Characterization of MSCs expressing islet neogenesis associated protein (INGAP): INGAP secretion and cell survival in vitro and in vivo.Heliyon. 2024 Jul 31;10(15):e35372. doi: 10.1016/j.heliyon.2024.e35372. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170459 Free PMC article.
-
Effective endothelial cell and human pluripotent stem cell interactions generate functional insulin-producing beta cells.Diabetologia. 2016 Nov;59(11):2378-2386. doi: 10.1007/s00125-016-4078-1. Epub 2016 Aug 27. Diabetologia. 2016. PMID: 27567623 Free PMC article.
References
-
- Cerasi E, Boitard C, Efendic S, et al. The islet in type 2 diabetes: back to center stage. Diabetes. 2001;50(suppl 1):S1–S3. - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- UK Prospective Diabetes Study Group. UK prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. - PubMed
-
- Bouwens L. Transdifferentiation versus stem cell hypothesis for the regeneration of islet beta-cells in the pancreas. Microsc Res Tech. 1998;43:332–336. - PubMed
-
- Hellerstrom C, Swenne I. Growth patterns of pancreatic islets in animals. In: Volk B, Arquillo E, eds. The Diabetic Pancreas. New York: Plenum Medical; 1985:53–80.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

