Differences between the negatively activating potassium conductances of Mammalian cochlear and vestibular hair cells
- PMID: 15492886
- PMCID: PMC2504553
- DOI: 10.1007/s10162-004-4051-4
Differences between the negatively activating potassium conductances of Mammalian cochlear and vestibular hair cells
Abstract
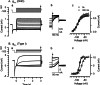
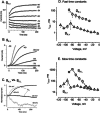
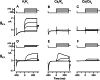
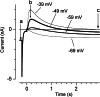
Cochlear and type I vestibular hair cells of mammals express negatively activating potassium (K(+)) conductances, called g(K,n) and g(K,L) respectively, which are important in setting the hair cells' resting potentials and input conductances. It has been suggested that the channels underlying both conductances include KCNQ4 subunits from the KCNQ family of K(+) channels. In whole-cell recordings from rat hair cells, we found substantial differences between g(K,n) and g(K,L) in voltage dependence, kinetics, ionic permeability, and stability during whole-cell recording. Relative to g(K,L), g(K,n) had a significantly broader and more negative voltage range of activation and activated with less delay and faster principal time constants over the negative part of the activation range. Deactivation of g(K,n) had an unusual sigmoidal time course, while g(K,L) deactivated with a double-exponential decay. g(K,L), but not g(K,n), had appreciable permeability to Cs(+). Unlike g(K,L), g(K,n)'s properties did not change ("wash out") during the replacement of cytoplasmic solution with pipette solution during ruptured-patch recordings. These differences in the functional expression of g(K,n) and g(K,L) channels suggest that there are substantial differences in their molecular structure as well.
Figures


Similar articles
-
A delayed rectifier conductance in type I hair cells of the mouse utricle.J Neurophysiol. 1996 Aug;76(2):995-1004. doi: 10.1152/jn.1996.76.2.995. J Neurophysiol. 1996. PMID: 8871214
-
Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista.J Neurophysiol. 2003 Jul;90(1):155-64. doi: 10.1152/jn.00244.2003. J Neurophysiol. 2003. PMID: 12843307
-
Resting potential and submembrane calcium concentration of inner hair cells in the isolated mouse cochlea are set by KCNQ-type potassium channels.J Neurosci. 2003 Mar 15;23(6):2141-9. doi: 10.1523/JNEUROSCI.23-06-02141.2003. J Neurosci. 2003. PMID: 12657673 Free PMC article.
-
Channeling your inner ear potassium: K(+) channels in vestibular hair cells.Hear Res. 2016 Aug;338:40-51. doi: 10.1016/j.heares.2016.01.015. Epub 2016 Feb 4. Hear Res. 2016. PMID: 26836968 Review.
-
K+ cycling and the endocochlear potential.Hear Res. 2002 Mar;165(1-2):1-9. doi: 10.1016/s0378-5955(02)00279-4. Hear Res. 2002. PMID: 12031509 Review.
Cited by
-
An allosteric gating model recapitulates the biophysical properties of IK,L expressed in mouse vestibular type I hair cells.J Physiol. 2017 Nov 1;595(21):6735-6750. doi: 10.1113/JP274202. Epub 2017 Sep 24. J Physiol. 2017. PMID: 28862328 Free PMC article.
-
Ca(2+) currents and voltage responses in Type I and Type II hair cells of the chick embryo semicircular canal.Pflugers Arch. 2005 Nov;451(2):395-408. doi: 10.1007/s00424-005-1466-7. Epub 2005 Aug 16. Pflugers Arch. 2005. PMID: 16133262
-
M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle.J Neurosci. 2006 Oct 4;26(40):10253-69. doi: 10.1523/JNEUROSCI.2596-06.2006. J Neurosci. 2006. PMID: 17021181 Free PMC article.
-
K+ Accumulation and Clearance in the Calyx Synaptic Cleft of Type I Mouse Vestibular Hair Cells.Neuroscience. 2020 Feb 1;426:69-86. doi: 10.1016/j.neuroscience.2019.11.028. Epub 2019 Dec 14. Neuroscience. 2020. PMID: 31846752 Free PMC article.
-
Simultaneous recordings from vestibular Type I hair cells and their calyceal afferents in mice.Front Neurol. 2024 Aug 27;15:1434026. doi: 10.3389/fneur.2024.1434026. eCollection 2024. Front Neurol. 2024. PMID: 39263277 Free PMC article.
References
-
- Angelaki DE. Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. III. Responses to translation. J. Neurophysiol. 1998;80:680–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

