Reduced inhibitory action of a GABAB receptor agonist on [3H]-dopamine release from rat ventral tegmental area in vitro after chronic nicotine administration
- PMID: 15494079
- PMCID: PMC526276
- DOI: 10.1186/1471-2210-4-24
Reduced inhibitory action of a GABAB receptor agonist on [3H]-dopamine release from rat ventral tegmental area in vitro after chronic nicotine administration
Abstract
Background: The activation of GABAB receptors in the ventral tegmental area (VTA) has been suggested to attenuate the rewarding properties of psychostimulants, including nicotine. However, the neurochemical mechanism that underlie this effect remains unknown. Since GABAB receptors modulate the release of several neurotransmitters in the mammalian brain, we have characterised the effect of the GABAB receptor agonist baclofen on the release of [3H]-dopamine ([3H]-DA) from VTA slices of naive rats and of rats pre-treated with nicotine.
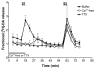
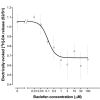
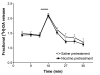
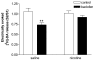
Results: In naive rats, baclofen concentration-dependently inhibited the electrically evoked release of [3H]-DA from the isolated VTA (EC50 = 0.103 microM, 95% CI = 0.043-0.249), without affecting the basal [3H]-monoamine overflow. This effect was mediated by activation of GABAB receptors as it was blocked by the selective receptor antagonist CGP55845A. Chronic administration of nicotine (0.4 mg kg(-1), s.c., for 14 days) affected neither the basal nor the electrically evoked release of [3H]-DA from VTA slices. However, the inhibitory effect of baclofen (10 microM) on the stimulated [3H]-monoamine overflow was abolished in rats pre-treated with nicotine as compared to saline-injected controls.
Conclusions: Our results demonstrate that GABAB receptor activation reduces the release of DA from the rat VTA. In addition, a reduced sensitivity of VTA GABAB receptors appears to develop after chronic exposure to nicotine. The resulting disinhibition of VTA DA neurones might therefore contribute to the sensitised dopaminergic responses observed in the rat mesocorticolimbic system following repeated administration of nicotine.
Figures
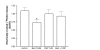
Similar articles
-
Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area.Synapse. 1994 Jan;16(1):36-44. doi: 10.1002/syn.890160105. Synapse. 1994. PMID: 8134899
-
Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis.Brain Res. 2004 Sep 17;1021(1):82-91. doi: 10.1016/j.brainres.2004.06.053. Brain Res. 2004. PMID: 15328035
-
GABA-A and GABA-B receptors mediate feeding elicited by the GABA-B agonist baclofen in the ventral tegmental area and nucleus accumbens shell in rats: reciprocal and regional interactions.Brain Res. 2010 Oct 8;1355:86-96. doi: 10.1016/j.brainres.2010.07.109. Epub 2010 Aug 7. Brain Res. 2010. PMID: 20696149
-
Effect of nicotine on dynamic function of brain catecholamine neurons.Ciba Found Symp. 1990;152:169-80; discussion 180-5. doi: 10.1002/9780470513965.ch10. Ciba Found Symp. 1990. PMID: 2209253 Review.
-
Suppressing Effect of Baclofen on Multiple Alcohol-Related Behaviors in Laboratory Animals.Front Psychiatry. 2018 Sep 28;9:475. doi: 10.3389/fpsyt.2018.00475. eCollection 2018. Front Psychiatry. 2018. PMID: 30323777 Free PMC article. Review.
Cited by
-
Dopamine D₂ and acetylcholine α7 nicotinic receptors have subcellular distributions favoring mediation of convergent signaling in the mouse ventral tegmental area.Neuroscience. 2013 Nov 12;252:126-43. doi: 10.1016/j.neuroscience.2013.08.008. Epub 2013 Aug 15. Neuroscience. 2013. PMID: 23954803 Free PMC article.
-
GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons.Neuroscience. 2011 Jan 13;172:94-103. doi: 10.1016/j.neuroscience.2010.10.046. Epub 2010 Oct 23. Neuroscience. 2011. PMID: 20974231 Free PMC article.
-
Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats.J Neurochem. 2008 Jul;106(2):943-56. doi: 10.1111/j.1471-4159.2008.05456.x. Epub 2008 May 3. J Neurochem. 2008. PMID: 18466321 Free PMC article.
-
Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation.Neuropharmacology. 2014 Jan;76 Pt B(0 0):554-65. doi: 10.1016/j.neuropharm.2013.05.042. Epub 2013 Jun 7. Neuropharmacology. 2014. PMID: 23752091 Free PMC article. Review.
-
The "stop" and "go" of nicotine dependence: role of GABA and glutamate.Cold Spring Harb Perspect Med. 2013 Jun 1;3(6):a012146. doi: 10.1101/cshperspect.a012146. Cold Spring Harb Perspect Med. 2013. PMID: 23732855 Free PMC article. Review.
References
-
- Corrigall WA, Franklin KJB, Coen KM, Clarke PBS. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

