The GAT domains of clathrin-associated GGA proteins have two ubiquitin binding motifs
- PMID: 15494413
- PMCID: PMC2911622
- DOI: 10.1074/jbc.M406654200
The GAT domains of clathrin-associated GGA proteins have two ubiquitin binding motifs
Abstract
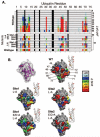
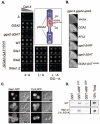
Ubiquitin (Ub) attachment to membrane proteins can serve as a sorting signal for lysosomal delivery. Recognition of Ub as a sorting signal can occur at the trans-Golgi network and is mediated in part by the clathrin-associated Golgi-localizing, gamma-adaptin ear domain homology, ARF-binding proteins (GGA). GGA proteins bind Ub via a three-helix bundle subdomain in their GAT (GGA and target of Myb1 protein) domain, which is also present in the Ub binding domain of target of Myb1 protein. Ubiquitin binding by yeast Ggas is required to direct sorting of ubiquitinated proteins such as general amino acid permease (Gap1) from the trans-Golgi network to endosomes. Using affinity chromatography and nuclear magnetic resonance spectroscopy, we have found that the human GGA3 GAT domain contains two Ub binding motifs that bind to the same surface of ubiquitin. These motifs are found within different helices within the three-helix GAT subdomain. When functionally analyzed in yeast, each motif was sufficient to mediate trans-Golgi network to endosomal sorting of Gap1, and mutation of both motifs resulted in defective Gap1 sorting without defects in other GGA-dependent processes.
Figures


References
-
- Hicke L, Dunn R. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. - PubMed
-
- Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L. Nat. Cell Biol. 2002;4:389–393. - PubMed
-
- Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP. Nature. 2002;416:451–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

