Buccal or sublingual misoprostol for cervical ripening and induction of labour
- PMID: 15495088
- PMCID: PMC8768472
- DOI: 10.1002/14651858.CD004221.pub2
Buccal or sublingual misoprostol for cervical ripening and induction of labour
Abstract
Background: This is one of a series of reviews of cervical ripening and labour induction using standardised methodology. Misoprostol administered by the oral and sublingual routes have the advantage of rapid onset of action, while the sublingual and vaginal routes have the advantage of prolonged activity and greatest bioavailability.
Objectives: To determine the effectiveness and safety of misoprostol administered buccally or sublingually for third trimester cervical ripening and induction of labour.
Search strategy: We searched the Cochrane Pregnancy and Childbirth Group trials register (8 December 2003), the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 4, 2003), and bibliographies of relevant papers.
Selection criteria: Randomised controlled trials comparing buccal or sublingual misoprostol used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis: A generic strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. Data were extracted onto standardized forms, checked for accuracy, and analysed using RevMan software.
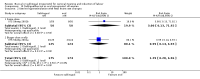
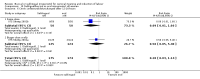
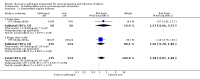
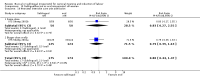
Main results: Three studies (502 participants) compared buccal/sublingual misoprostol respectively with a vaginal regimen (200 microg versus 50 microg) and with oral administration (50 versus 50 microg and 50 versus 100microg).The buccal route was associated with a trend to fewer caesarean sections than with the vaginal route (18/73 versus 28/79; relative risk (RR) 0.70; 95% confidence interval (CI) 0.42 to 1.15). There were no significant differences in any other outcomes. When the same dosage was used sublingually versus orally, the sublingual route was associated with less failures to achieve vaginal delivery within 24 hours (12/50 versus 19/50; RR 0.63, 95% CI 0.34 to 1.16), reduced oxytocin augmentation (17/50 versus 23/50; RR 0.74, 95% CI 0.45 to 1.21) and reduced caesarean section (8/50 versus 15/50; RR 0.53, 95% CI 0.25 to 1.14), but the differences were not statistically significant. When a smaller dose was used sublingually than orally, there were no differences in any of the outcomes.
Reviewers' conclusions: Based on only three small trials, sublingual misoprostol appears to be at least as effective as when the same dose is administered orally. There are inadequate data to comment on the relative complications and side-effects. Sublingual or buccal misoprostol should not enter clinical use until its safety and optimal dosage have been established by larger trials.
Conflict of interest statement
None known.
Figures

















Update of
References
References to studies included in this review
075 Shetty 2002a {published data only}
-
- Shetty A, Mackie L, Danielian P, Rice P, Templeton A. Sublingual compared with oral misoprostol in term labour induction: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2002;109:645‐50. - PubMed
075 Shetty 2002b {published data only}
-
- Shetty A, Danielian P, Templeton A. Sublingual misoprostol for the induction of labor at term. American Journal of Obstetrics and Gynecology 2002;186(1):72‐6. - PubMed
-
- Shetty A, Danielian P, Templeton A. Sublingual misoprostol in the induction of labour at term [abstract]. Journal of Obstetrics and Gynaecology 2001;21 Suppl 1:S51.
200 Carlan 2002 {published data only}
-
- Carlan S, Blust D, OBrien W. Buccal versus intravaginal misoprostol administration for cervical ripening. American Journal of Obstetrics and Gynecology 2002;186:229‐33. - PubMed
References to studies excluded from this review
Todd 2002 {published data only}
-
- Todd CS, Soler M, Castleman L, Rogers MK, Blumenthal PD. Buccal misoprostol as cervical preparation for second trimester pregnancy termination. Contraception 2002;65(6):415‐8. - PubMed
References to studies awaiting assessment
Amador 2007 {published data only}
-
- Amador LAV, Carmona JCF, Gallego FG, Texido CS, Esteve JLC. Randomized clinical trial of the safety and efficacy of 50 microg sublingual misoprostol versus 25 microg vaginal misoprostol for labor induction at term in pregnant women with diabetes. Progresos de Obstetricia y Ginecologia 2007;50(8):473‐83.
Azeem 2006 {published data only}
-
- Azeem S. Buccal vs. intravaginal misoprostol administration for cervical ripening in induction of labor [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:95.
Bartusevicius 2006 {published data only}
-
- Bartusevicius A, Barcaite E, Krikstolaitis R, Gintautas V, Nadisauskiene R. Sublingual compared with vaginal misoprostol for labour induction at term: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2006;113:1431‐7. - PubMed
Caliskan 2005 {published data only}
-
- Caliskan E, Bodur H, Ozeren S, Corakci A, Ozkan S, Yucesoy I. Misoprostol 50 [mu]g sublingually versus vaginally for labor induction at term: a randomized study. Gynecologic and Obstetric Investigation 2005;59(3):155‐61. - PubMed
Elhassan 2007 {published data only}
-
- Elhassan EM, Nasr AM, Adam I. Sublingual compared with oral and vaginal misoprostol for labor induction. International Journal of Gynecology & Obstetrics 2007;97(2):153‐4. - PubMed
Esteve 2006 {published data only}
-
- Esteve JLC, Garcia TJP, Iturralde AS, Ferrer YA, Teixido CS. Randomized, controlled clinical trial to evaluate the safety and efficacy of 25 microg of vaginal misoprostol versus 50 microg of sublingual misoprostol for labor induction [Ensayo clinico, aleatorizado y controlado para evaluar la eficacia y la seguridad de la administracion de 25 mug de misoprostol vaginal frente a 50 mug de misoprostol sublingual para la induccion del trabajo del parto]. Progresos de Obstetricia y Ginecologia 2006;49(7):369‐79.
Feitosa 2006 {published data only}
-
- Feitosa F. Sublingual versus vaginal misoprostol for induction of labor [abstract]. Revista Brasileira de Ginecologia y Obstetricia 2006;28(9):566.
Feitosa 2006a {published data only}
-
- Feitosa FE, Sampaio ZS, Alencar CA, Amorim MM, Passini R. Sublingual vs. vaginal misoprostol for induction of labor. International Journal of Gynecology & Obstetrics 2006;94(2):91‐5. - PubMed
Filho 2005 {published data only}
-
- Filho O, Albuquerque R, Pacheco A, et al. Sublingual versus vaginal misoprostol for labor induction of term pregnancies. Revista Brasileira de Ginecologia e Obstetricia 2005;27(1):24‐31.
Lo 2006 {published data only}
-
- Lo TK, Lau WL, Wong KS, Tang LC. Sublingual misoprostol compared to artificial rupture of membranes plus oxytocin infusion for labour induction in nulliparous women with a favourable cervix at term. Hong Kong Medical Journal 2006;12(5):345‐50. - PubMed
Nassar 2006 {published data only}
-
- Nassar AH. Sublingual versus vaginal misoprostol for labor induction at term (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
Nassar 2007 {published data only}
-
- Nassar AH, Awwad J, Khalil AM, Abu‐Musa A, Mehio G, Usta IM. A randomised comparison of patient satisfaction with vaginal and sublingual misoprostol for induction of labour at term. BJOG: an international journal of obstetrics and gynaecology 2007;114(10):1215‐21. - PubMed
Parisaei 2005 {published data only}
-
- Parisaei MP, Erskine KJE. Comparison of sub‐lingual misoprostol with standard regime vaginal prostaglandin E2 gel for the induction of labour after term rupture of membranes [abstract]. Journal of Obstetrics and Gynaecology 2005;25 Suppl 1:S69.
Parisaei 2008 {published data only}
-
- Parisaei M, Erskine KJ. Is expensive always better? Comparison of two induction agents for term rupture of membranes. Journal of Obstetrics and Gynaecology 2008;28(3):290‐3. - PubMed
Penaranda 2002 {published data only}
-
- Penaranda W, Arrieta O, Yances B. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en al hospital de maternidad rafael calvo de cartagen]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87‐92.
Russell 2007 {published data only}
-
- Russell Z, O'Leary T, Destefano K, Deutsch A, Carlan S. Buccal versus vaginal misoprostol administration for cervical ripening. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S37, Abstract no: 85.
Wolf 2005 {published data only}
-
- Wolf SB, Sanchez‐Ramos L, Kaunitz AM. Sublingual misoprostol for labor induction: a randomized clinical trial. Obstetrics & Gynecology 2005;105:365‐71. - PubMed
Additional references
Alfirevic 2003
-
- Alfirevic Z. Oral misoprostol for induction of labour (Cochrane Review). The Cochrane Library 2003, Issue 1. - PubMed
Clarke 2000
-
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1 Oxford, England: The Cochrane Collaboration, 2000.
Curtis 1987
-
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. - PubMed
Danielsson 1999
-
- Danielsson KG, Marions L, Rodriguez A, Spur BW, Wong PY, Bygdeman M. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstetrics & Gynecology 1999;93:275‐80. - PubMed
Hofmeyr 1998
-
- Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105:971‐5. - PubMed
Hofmeyr 1999
-
- Hofmeyr GJ, Gulmezoglu AM, Alfirevic Z. Misoprostol for induction of labour: a systematic review. British Journal of Obstetrics and Gynaecology 1999;106:798‐803. - PubMed
Hofmeyr 2003a
-
- Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol (Protocol for a Cochrane Review). The Cochrane Library 2003, Issue 1.
Hofmeyr 2003b
-
- Hofmeyr GJ, Gulmezoglu AM. Vaginal misoprostol for cervical ripening and labour induction in late pregnancy (Cochrane Review). The Cochrane Library 2003, Issue 1. - PubMed
RevMan 2002 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
Shetty 2002
-
- Shetty A, Danielian P, Templeton A. Sublingual misoprostol for the induction of labour at term. American Journal of Obstetrics and Gynecology 2002;186:72‐6. - PubMed
Tang 2002
-
- Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Human Reproduction 2002;17:332‐6. - PubMed
Zieman 1997
-
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

