Identification and characterization of the human ARD1-NATH protein acetyltransferase complex
- PMID: 15496142
- PMCID: PMC1134861
- DOI: 10.1042/BJ20041071
Identification and characterization of the human ARD1-NATH protein acetyltransferase complex
Abstract
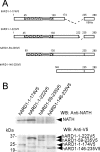
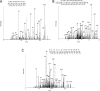
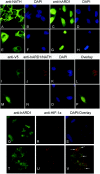
Protein acetyltransferases and deacetylases have been implicated in oncogenesis, apoptosis and cell cycle regulation. Most of the protein acetyltransferases described acetylate epsilon-amino groups of lysine residues within proteins. Mouse ARD1 (homologue of yeast Ard1p, where Ard1p stands for arrest defective 1 protein) is the only known protein acetyltransferase catalysing acetylation of proteins at both alpha-(N-terminus) and epsilon-amino groups. Yeast Ard1p interacts with Nat1p (N-acetyltransferase 1 protein) to form a functional NAT (N-acetyltransferase). We now describe the human homologue of Nat1p, NATH (NAT human), as the partner of the hARD1 (human ARD1) protein. Included in the characterization of the NATH and hARD1 proteins is the following: (i) endogenous NATH and hARD1 proteins are expressed in human epithelial, glioma and promyelocytic cell lines; (ii) NATH and hARD1 form a stable complex, as investigated by reciprocal immunoprecipitations followed by MS analysis; (iii) NATH-hARD1 complex expresses N-terminal acetylation activity; (iv) NATH and hARD1 interact with ribosomal subunits, indicating a co-translational acetyltransferase function; (v) NATH is localized in the cytoplasm, whereas hARD1 localizes both to the cytoplasm and nucleus; (vi) hARD1 partially co-localizes in nuclear spots with the transcription factor HIF-1alpha (hypoxia-inducible factor 1alpha), a known epsilon-amino substrate of ARD1; (vii) NATH and hARD1 are cleaved during apoptosis, resulting in a decreased NAT activity. This study identifies the human homologues of the yeast Ard1p and Nat1p proteins and presents new aspects of the NATH and hARD1 proteins relative to their yeast homologues.
Figures


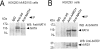


References
-
- Cohen H. Y., Lavu S., Bitterman K. J., Hekking B., Imahiyerobo T. A., Miller C., Frye R., Ploegh H., Kessler B. M., Sinclair D. A. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell. 2004;13:627–638. - PubMed
-
- Fu M., Wang C., Wang J., Zafonte B. T., Lisanti M. P., Pestell R. G. Acetylation in hormone signaling and the cell cycle. Cytokine Growth Factor Rev. 2002;13:259–276. - PubMed
-
- Di Gennaro E., Bruzzese F., Caraglia M., Abruzzese A., Budillon A. Acetylation of proteins as novel target for antitumor therapy: review article. Amino Acids. 2004;26:435–441. - PubMed
-
- Sugiura N., Adams S. M., Corriveau R. A. An evolutionarily conserved N-terminal acetyltransferase complex associated with neuronal development. J. Biol. Chem. 2003;278:40113–40120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

