Concurrent administration of donepezil HCl and levodopa/carbidopa in patients with Parkinson's disease: assessment of pharmacokinetic changes and safety following multiple oral doses
- PMID: 15496222
- PMCID: PMC1884553
- DOI: 10.1111/j.1365-2125.2004.01799.x
Concurrent administration of donepezil HCl and levodopa/carbidopa in patients with Parkinson's disease: assessment of pharmacokinetic changes and safety following multiple oral doses
Abstract
Aim: The use of acetylcholinesterase inhibitors for the treatment of comorbid Alzheimer's disease in Parkinson's disease (PD) patients stabilized on a levodopa regimen may potentially disrupt cholinergic balance. This randomized, double-blind, crossover study investigated the safety of, and possible drug-drug interaction between, donepezil HCl and levodopa/carbidopa.
Methods: Twenty-five patients with PD who were taking physician-optimized doses of levodopa/carbidopa (with daytime dosing intervals of 4-8 h) were administered once-daily doses of either donepezil HCl (5 mg) or placebo for 15 days, in two treatment periods, separated by a washout of at least 2 weeks. Some patients took a second dose of levodopa/carbidopa after 4 h, therefore subanalysis of the levodopa/carbidopa data was conducted up to 4 h and 8 h after dosing. Twenty-six healthy matched controls received open-label donepezil HCl only, for a single 15-day period. Blood samples were collected before, during and after the 15 doses of donepezil HCl for pharmacokinetic (PK) assessments. Pharmacokinetic parameters included maximum attained plasma drug concentration (C(max)), time at which C(max) is attained (t(max)), plasma drug concentration at steady state (C(ss)), and area under the drug concentration-time curve over the dosing interval. Safety assessments included monitoring adverse events, and the Unified Parkinson's Disease Rating Scale (UPDRS) motor examination.
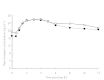
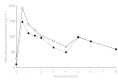
Results: The mean age of all subjects was 72.6 +/- 1.3 years. Donepezil PK assessments of PD patients receiving levodopa/carbidopa were similar to the PK results from healthy controls who received donepezil HCl only (mean AUC(0-12 h)= 281.6 +/- 17.6 and 268.6 +/- 19.9 ng.h ml(-1), respectively). Carbidopa PK were not significantly altered by the concomitant administration of multiple doses of donepezil HCl, compared with when PD patients received placebo (mean AUC(0-8 h)= 921.8 +/- 160 and 821.8 +/- 113 ng.h ml(-1), respectively). Four hours after administration of donepezil HCl in PD patients, AUC(0-4 h), C(max) and C(ss) of levodopa were higher than when PD patients received placebo (P < 0.05). Eight hours after donepezil HCl, however, only C(max) and t(max) were observed to change compared with when PD patients received placebo (mean C(max) = 2652 +/- 429 and 2077 +/- 276 ng ml(-1), respectively; mean t(max) = 1.7 +/- 0.4 and 2.9 +/- 0.5 h, respectively; P< or = 0.05). The number of PD patients who experienced at least one adverse event during the study (13/25) was higher when they received donepezil HCl than when they received placebo (5/25), but was the same as healthy subjects who received donepezil HCl only (13/26). There were no significant differences in change from baseline on the UPDRS motor examination parameters in PD patients when they took donepezil HCl and when they took placebo.
Conclusions: No clinically significant drug-drug interactions between donepezil HCl and levodopa/carbidopa were observed at steady state. The small changes in the pharmacokinetics of levodopa did not result in any change in motor symptoms. Co-administration of the two drugs led to a small increase in adverse events compared with administration of levodopa/carbidopa alone in PD patients. These adverse events, however, were consistent with donepezil's cholinomimetic effect, and their incidence was comparable to that observed following the administration of donepezil HCl alone.
Figures
Similar articles
-
Concurrent administration of donepezil HCl and risperidone in patients with schizophrenia: assessment of pharmacokinetic changes and safety following multiple oral doses.Br J Clin Pharmacol. 2004 Nov;58 Suppl 1(Suppl 1):50-7. doi: 10.1111/j.1365-2125.2004.01817.x. Br J Clin Pharmacol. 2004. PMID: 15496223 Free PMC article. Clinical Trial.
-
A multicenter, open-label, sequential study comparing preferences for carbidopa-levodopa orally disintegrating tablets and conventional tablets in subjects with Parkinson's disease.Clin Ther. 2005 Jan;27(1):58-63. doi: 10.1016/j.clinthera.2005.01.004. Clin Ther. 2005. PMID: 15763606 Clinical Trial.
-
Pharmacokinetic-pharmacodynamic interaction between nebicapone and controlled-release levodopa/benserazide: a single-center, Phase I, double-blind, randomized, placebo-controlled, four-way crossover study in healthy subjects.Clin Ther. 2009 Oct;31(10):2258-71. doi: 10.1016/j.clinthera.2009.10.019. Clin Ther. 2009. PMID: 19922897 Clinical Trial.
-
Carbidopa/Levodopa ER Capsules (Rytary(®), Numient™): A Review in Parkinson's Disease.CNS Drugs. 2016 Jan;30(1):79-90. doi: 10.1007/s40263-015-0306-3. CNS Drugs. 2016. PMID: 26692288 Review.
-
Pharmacokinetic considerations for the use of levodopa in the treatment of Parkinson disease: focus on levodopa/carbidopa/entacapone for treatment of levodopa-associated motor complications.Clin Neuropharmacol. 2013 May-Jun;36(3):84-91. doi: 10.1097/WNF.0b013e31828f3385. Clin Neuropharmacol. 2013. PMID: 23673910 Review.
Cited by
-
Community and long-term care management of Parkinson's disease in the elderly: focus on monoamine oxidase type B inhibitors.Drugs Aging. 2007;24(8):663-80. doi: 10.2165/00002512-200724080-00004. Drugs Aging. 2007. PMID: 17702535 Review.
-
Cholinesterase Inhibitors for Lewy Body Disorders: A Meta-Analysis.Int J Neuropsychopharmacol. 2015 Jul 28;19(2):pyv086. doi: 10.1093/ijnp/pyv086. Int J Neuropsychopharmacol. 2015. PMID: 26221005 Free PMC article. Review.
-
The spectrum of cognitive impairment in Lewy body diseases.Mov Disord. 2014 Apr 15;29(5):608-21. doi: 10.1002/mds.25866. Mov Disord. 2014. PMID: 24757110 Free PMC article. Review.
-
Effect of levodopa on cognitive function in Parkinson's disease with and without dementia and dementia with Lewy bodies.J Neurol Neurosurg Psychiatry. 2006 Dec;77(12):1323-8. doi: 10.1136/jnnp.2006.098079. Epub 2006 Sep 4. J Neurol Neurosurg Psychiatry. 2006. PMID: 16952917 Free PMC article. Clinical Trial.
-
Release behaviour and toxicity evaluation of levodopa from carboxylated single-walled carbon nanotubes.Beilstein J Nanotechnol. 2015 Jan 22;6:243-53. doi: 10.3762/bjnano.6.23. eCollection 2015. Beilstein J Nanotechnol. 2015. PMID: 25671168 Free PMC article.
References
-
- Davies P, Maloney AJF. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. - PubMed
-
- Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. - PubMed
-
- Bowen DM, Benton JS, Spillane JA, Smith CC, Allen SJ. Choline acetyltransferase activity and histopathology of frontal neocortex from biopsies of demented patients. J Neurol Sci. 1982;57:191–202. - PubMed
-
- Rogers SL, Yamanishi Y, Yamatsu K. E2020: the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 314–20.
-
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer's disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

