Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes
- PMID: 15496442
- PMCID: PMC3882150
- DOI: 10.1242/dev.01437
Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes
Abstract
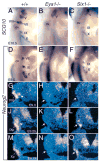
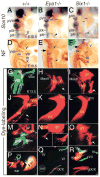
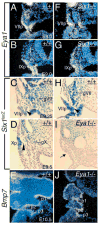
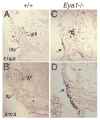
Eya1 encodes a transcriptional co-activator and is expressed in cranial sensory placodes. It interacts with and functions upstream of the homeobox gene Six1 during otic placodal development. Here, we have examined their role in cranial sensory neurogenesis. Our data show that the initial cell fate determination for the vestibuloacoustic neurons and their delamination appeared to be unaffected in the absence of Eya1 or Six1 as judged by the expression of the basic helix-loop-helix genes, Neurog1 that specifies the neuroblast cell lineage, and Neurod that controls neuronal differentiation and survival. However, both genes are necessary for normal maintenance of neurogenesis. During the development of epibranchial placode-derived distal cranial sensory ganglia, while the phenotype appears less severe in Six1 than in Eya1 mutants, an early arrest of neurogenesis was observed in the mutants. The mutant epibranchial progenitor cells fail to express Neurog2 that is required for the determination of neuronal precursors, and other basic helix-loop-helix as well as the paired homeobox Phox2 genes that are essential for neural differentiation and maintenance. Failure to activate their normal differentiation program resulted in abnormal apoptosis of the progenitor cells. Furthermore, we show that disruption of viable ganglion formation leads to pathfinding errors of branchial motoneurons. Finally, our results suggest that the Eya-Six regulatory hierarchy also operates in the epibranchial placodal development. These findings uncover an essential function for Eya1 and Six1 as critical determination factors in acquiring both neuronal fate and neuronal subtype identity from epibranchial placodal progenitors. These analyses define a specific role for both genes in early differentiation and survival of the placodally derived cranial sensory neurons.
Figures




Similar articles
-
Six1 and Eya1 both promote and arrest neuronal differentiation by activating multiple Notch pathway genes.Dev Biol. 2017 Nov 15;431(2):152-167. doi: 10.1016/j.ydbio.2017.09.027. Epub 2017 Sep 22. Dev Biol. 2017. PMID: 28947179
-
The role of Six1 in mammalian auditory system development.Development. 2003 Sep;130(17):3989-4000. doi: 10.1242/dev.00628. Development. 2003. PMID: 12874121 Free PMC article.
-
Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1.Dev Biol. 2006 May 15;293(2):499-512. doi: 10.1016/j.ydbio.2005.12.015. Epub 2006 Mar 10. Dev Biol. 2006. PMID: 16530750 Free PMC article.
-
Induction and specification of cranial placodes.Dev Biol. 2006 Jun 15;294(2):303-51. doi: 10.1016/j.ydbio.2006.03.009. Epub 2006 May 3. Dev Biol. 2006. PMID: 16677629 Review.
-
Making senses development of vertebrate cranial placodes.Int Rev Cell Mol Biol. 2010;283:129-234. doi: 10.1016/S1937-6448(10)83004-7. Int Rev Cell Mol Biol. 2010. PMID: 20801420 Review.
Cited by
-
Six1 promotes proliferation of pancreatic cancer cells via upregulation of cyclin D1 expression.PLoS One. 2013;8(3):e59203. doi: 10.1371/journal.pone.0059203. Epub 2013 Mar 20. PLoS One. 2013. PMID: 23527134 Free PMC article.
-
Cells, molecules and morphogenesis: the making of the vertebrate ear.Brain Res. 2006 May 26;1091(1):151-71. doi: 10.1016/j.brainres.2006.02.078. Epub 2006 Apr 27. Brain Res. 2006. PMID: 16643865 Free PMC article. Review.
-
The role of foxi family transcription factors in the development of the ear and jaw.Curr Top Dev Biol. 2015;111:461-95. doi: 10.1016/bs.ctdb.2014.11.014. Epub 2015 Jan 21. Curr Top Dev Biol. 2015. PMID: 25662269 Free PMC article. Review.
-
Setting appropriate boundaries: fate, patterning and competence at the neural plate border.Dev Biol. 2014 May 1;389(1):2-12. doi: 10.1016/j.ydbio.2013.11.027. Epub 2013 Dec 7. Dev Biol. 2014. PMID: 24321819 Free PMC article. Review.
-
Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit.Dev Dyn. 2005 Nov;234(3):633-50. doi: 10.1002/dvdy.20551. Dev Dyn. 2005. PMID: 16145671 Free PMC article.
References
-
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, et al. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15:157–164. - PubMed
-
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. - PubMed
-
- Ayer-Le Lievre CS, Le Douarin NM. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Dev Biol. 1982;94:291–310. - PubMed
-
- Begbie J, Brunet JF, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. - PubMed
-
- Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

