Dynamic sound localization during rapid eye-head gaze shifts
- PMID: 15496665
- PMCID: PMC6730098
- DOI: 10.1523/JNEUROSCI.2671-04.2004
Dynamic sound localization during rapid eye-head gaze shifts
Erratum in
- J Neurosci. 2004 Nov 3;24(44):following 10034
Abstract
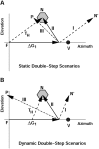
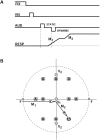
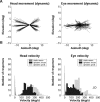
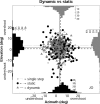
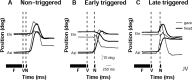
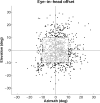
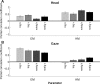
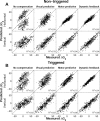
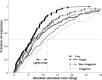
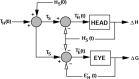
Human sound localization relies on implicit head-centered acoustic cues. However, to create a stable and accurate representation of sounds despite intervening head movements, the acoustic input should be continuously combined with feedback signals about changes in head orientation. Alternatively, the auditory target coordinates could be updated in advance by using either the preprogrammed gaze-motor command or the sensory target coordinates to which the intervening gaze shift is made ("predictive remapping"). So far, previous experiments cannot dissociate these alternatives. Here, we study whether the auditory system compensates for ongoing saccadic eye and head movements in two dimensions that occur during target presentation. In this case, the system has to deal with dynamic changes of the acoustic cues as well as with rapid changes in relative eye and head orientation that cannot be preprogrammed by the audiomotor system. We performed visual-auditory double-step experiments in two dimensions in which a brief sound burst was presented while subjects made a saccadic eye-head gaze shift toward a previously flashed visual target. Our results show that localization responses under these dynamic conditions remain accurate. Multiple linear regression analysis revealed that the intervening eye and head movements are fully accounted for. Moreover, elevation response components were more accurate for longer-duration sounds (50 msec) than for extremely brief sounds (3 msec), for all localization conditions. Taken together, these results cannot be explained by a predictive remapping scheme. Rather, we conclude that the human auditory system adequately processes dynamically varying acoustic cues that result from self-initiated rapid head movements to construct a stable representation of the target in world coordinates. This signal is subsequently used to program accurate eye and head localization responses.
Figures


Similar articles
-
Influence of head position on the spatial representation of acoustic targets.J Neurophysiol. 1999 Jun;81(6):2720-36. doi: 10.1152/jn.1999.81.6.2720. J Neurophysiol. 1999. PMID: 10368392
-
Gaze orienting in dynamic visual double steps.J Neurophysiol. 2005 Dec;94(6):4300-13. doi: 10.1152/jn.00027.2005. Epub 2005 Aug 17. J Neurophysiol. 2005. PMID: 16107519
-
Experimental test of spatial updating models for monkey eye-head gaze shifts.PLoS One. 2012;7(10):e47606. doi: 10.1371/journal.pone.0047606. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23118883 Free PMC article.
-
Behavioral studies of sound localization in the cat.J Neurosci. 1998 Mar 15;18(6):2147-60. doi: 10.1523/JNEUROSCI.18-06-02147.1998. J Neurosci. 1998. PMID: 9482800 Free PMC article. Review.
-
Multisensory guidance of orienting behavior.Hear Res. 2009 Dec;258(1-2):106-12. doi: 10.1016/j.heares.2009.05.008. Epub 2009 Jun 9. Hear Res. 2009. PMID: 19520151 Free PMC article. Review.
Cited by
-
Spatial localization of auditory stimuli in human auditory cortex is based on both head-independent and head-centered coordinate systems.J Neurosci. 2012 Sep 26;32(39):13501-9. doi: 10.1523/JNEUROSCI.1315-12.2012. J Neurosci. 2012. PMID: 23015439 Free PMC article.
-
Gaze shifts to auditory and visual stimuli in cats.J Assoc Res Otolaryngol. 2013 Oct;14(5):731-55. doi: 10.1007/s10162-013-0401-4. Epub 2013 Jun 8. J Assoc Res Otolaryngol. 2013. PMID: 23749194 Free PMC article.
-
Spectral Weighting Underlies Perceived Sound Elevation.Sci Rep. 2019 Feb 7;9(1):1642. doi: 10.1038/s41598-018-37537-z. Sci Rep. 2019. PMID: 30733476 Free PMC article.
-
Modeling sound-source localization in sagittal planes for human listeners.J Acoust Soc Am. 2014 Aug;136(2):791-802. doi: 10.1121/1.4887447. J Acoust Soc Am. 2014. PMID: 25096113 Free PMC article.
-
Modeling auditory-visual evoked eye-head gaze shifts in dynamic multisteps.J Neurophysiol. 2018 May 1;119(5):1795-1808. doi: 10.1152/jn.00502.2017. Epub 2018 Jan 31. J Neurophysiol. 2018. PMID: 29384452 Free PMC article.
References
-
- André-Deshays C, Berthoz A, Revel M (1988) Eye-head coupling in humans. I. Simultaneous recording of isolated motor units in dorsal neck muscles and horizontal eye movements. Exp Brain Res 69: 399-406. - PubMed
-
- Bahcall DO, Kowler E (1999) Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature 400: 864-866. - PubMed
-
- Blauert J (1997) Spatial hearing: the psychophysics of human sound localization. Cambridge, MA: MIT.
-
- Colby CL, Duhamel JR, Goldberg ME (1995) Oculocentric spatial representation in parietal cortex. Cereb Cortex 5: 470-481. - PubMed
-
- Collewijn H, van der Mark F, Jansen TC (1975) Precise recording of human eye movements. Vision Res 15: 447-450. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
