Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation
- PMID: 15496678
- PMCID: PMC6730095
- DOI: 10.1523/JNEUROSCI.2457-04.2004
Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation
Abstract
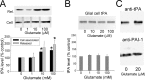
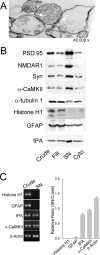
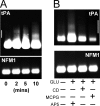
Long-term synaptic plasticity is both protein synthesis-dependent and synapse-specific. Therefore, the identity of the newly synthesized proteins, their localization, and mechanism of regulation are critical to our understanding of this process. Tissue plasminogen activator (tPA) is a secreted protease required for some forms of long-term synaptic plasticity. Here, we show tPA activity is rapidly increased in hippocampal neurons after glutamate stimulation. This increase in tPA activity corresponds to an increase in tPA protein synthesis that results from the translational activation of mRNA present at the time of stimulation. Furthermore, the mRNA encoding tPA is present in dendrites and is rapidly polyadenylated after glutamate stimulation. Both the polyadenylation of tPA mRNA and the subsequent increase in tPA protein is dependent on metabotropic glutamate receptor (mGluR) activation. A similar mGluR-dependent increase in tPA activity was detected after stimulation of a synaptic fraction isolated from the hippocampus, suggesting tPA synthesis is occurring in the synaptodendritic region. Finally, we demonstrate that tPA mRNA is bound by the mRNA-binding protein CPEB (cytoplasmic polyadenylation element binding protein-1), a protein known to regulate mRNA translation via polyadenylation. These results indicate that neurons are capable of synthesizing a secreted protein in the synaptic region, that mGluR activation induces mRNA polyadenylation and translation of specific mRNA, and suggest a model for synaptic plasticity whereby translational regulation of an immediate early gene precedes the increase in gene transcription.
Figures




References
-
- Baranes D, Lederfein D, Huang Y-Y, Chen M, Bailey CH, Kandel ER (1998) Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21: 813-825. - PubMed
-
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST (2001) Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107: 477-487. - PubMed
-
- Calabresi P, Napolitano M, Centonze D, Marfia GA, Gubellini P, Teule MA, Berretta N, Bernardi G, Frati L, Tolu M, Gulino A (2000) Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci 12: 1002-1012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
