Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis
- PMID: 15496985
- PMCID: PMC526462
- DOI: 10.1038/sj.emboj.7600445
Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis
Abstract
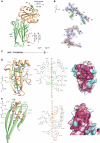
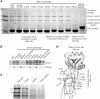
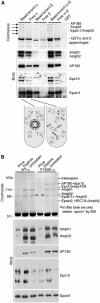
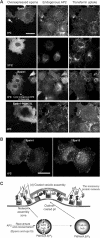
Clathrin-mediated endocytosis involves the assembly of a network of proteins that select cargo, modify membrane shape and drive invagination, vesicle scission and uncoating. This network is initially assembled around adaptor protein (AP) appendage domains, which are protein interaction hubs. Using crystallography, we show that FxDxF and WVxF peptide motifs from synaptojanin bind to distinct subdomains on alpha-appendages, called 'top' and 'side' sites. Appendages use both these sites to interact with their binding partners in vitro and in vivo. Occupation of both sites simultaneously results in high-affinity reversible interactions with lone appendages (e.g. eps15 and epsin1). Proteins with multiple copies of only one type of motif bind multiple appendages and so will aid adaptor clustering. These clustered alpha(appendage)-hubs have altered properties where they can sample many different binding partners, which in turn can interact with each other and indirectly with clathrin. In the final coated vesicle, most appendage binding partners are absent and thus the functional status of the appendage domain as an interaction hub is temporal and transitory giving directionality to vesicle assembly.
Figures




Similar articles
-
Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly.PLoS Biol. 2006 Sep;4(9):e262. doi: 10.1371/journal.pbio.0040262. PLoS Biol. 2006. PMID: 16903783 Free PMC article.
-
A novel AP-2 adaptor interaction motif initially identified in the long-splice isoform of synaptojanin 1, SJ170.J Biol Chem. 2004 Jan 16;279(3):2281-90. doi: 10.1074/jbc.M305644200. Epub 2003 Oct 17. J Biol Chem. 2004. PMID: 14565955
-
Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes.Science. 2001 Feb 9;291(5506):1051-5. doi: 10.1126/science.291.5506.1051. Science. 2001. PMID: 11161218
-
Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection.J Cell Biol. 2003 Oct 27;163(2):203-8. doi: 10.1083/jcb.200309175. J Cell Biol. 2003. PMID: 14581447 Free PMC article. Review.
-
Cargo recognition during clathrin-mediated endocytosis: a team effort.Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
-
Bending a membrane: how clathrin affects budding.Proc Natl Acad Sci U S A. 2006 Jun 6;103(23):8715-20. doi: 10.1073/pnas.0600312103. Epub 2006 May 30. Proc Natl Acad Sci U S A. 2006. PMID: 16735469 Free PMC article.
-
Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly.PLoS Biol. 2006 Sep;4(9):e262. doi: 10.1371/journal.pbio.0040262. PLoS Biol. 2006. PMID: 16903783 Free PMC article.
-
HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles.Mol Biol Cell. 2009 Nov;20(21):4563-74. doi: 10.1091/mbc.e09-04-0272. Epub 2009 Sep 9. Mol Biol Cell. 2009. PMID: 19741093 Free PMC article.
-
Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb.Cell. 2008 Sep 5;134(5):817-27. doi: 10.1016/j.cell.2008.07.023. Cell. 2008. PMID: 18775314 Free PMC article.
-
Systems biology and physical biology of clathrin-mediated endocytosis.Integr Biol (Camb). 2011 Aug;3(8):803-15. doi: 10.1039/c1ib00036e. Epub 2011 Jul 26. Integr Biol (Camb). 2011. PMID: 21792431 Free PMC article. Review.
References
-
- Brett TJ, Traub LM, Fremont DH (2002) Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure (Camb) 10: 797–809 - PubMed
-
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE (2001) Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol 17: 517–568 - PubMed
-
- Chen H, Slepnev VI, Di Fiore PP, De Camilli P (1999) The interaction of epsin and Eps15 with the clathrin adaptor AP-2 is inhibited by mitotic phosphorylation and enhanced by stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem 274: 3257–3260 - PubMed
-
- Collins BM, Praefcke GJ, Robinson MS, Owen DJ (2003) Structural basis for binding of accessory proteins by the appendage domain of GGAs. Nat Struct Biol 10: 607–613 - PubMed
-
- Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422: 37–44 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

