An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation
- PMID: 15496987
- PMCID: PMC526457
- DOI: 10.1038/sj.emboj.7600423
An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation
Abstract
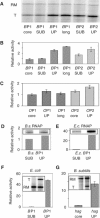
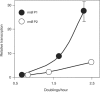
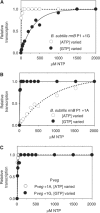
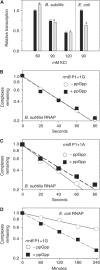
As an approach to the study of rRNA synthesis in Gram-positive bacteria, we characterized the regulation of the Bacillus subtilis rrnB and rrnO rRNA promoters. We conclude that B. subtilis and Escherichia coli use different strategies to control rRNA synthesis. In contrast to E. coli, it appears that the initiating NTP for transcription from B. subtilis rRNA promoters is GTP, promoter strength is determined primarily by the core promoter (-10/-35 region), and changes in promoter activity always correlate with changes in the intracellular GTP concentration. rRNA promoters in B. subtilis appear to be regulated by changes in the initiating NTP pools, but in some growth transitions, changes in rRNA promoter activity are also dependent on relA, which codes for ppGpp synthetase. In contrast to the situation for E. coli where ppGpp decreases rRNA promoter activity by directly inhibiting RNA polymerase, it appears that ppGpp may not inhibit B. subtilis RNA polymerase directly. Rather, increases in the ppGpp concentration might reduce the available GTP pools, thereby modulating rRNA promoter activity indirectly.
Figures



Similar articles
-
Physiological analysis of the stringent response elicited in an extreme thermophilic bacterium, Thermus thermophilus.J Bacteriol. 2006 Oct;188(20):7111-22. doi: 10.1128/JB.00574-06. J Bacteriol. 2006. PMID: 17015650 Free PMC article.
-
Kinetic properties of rrn promoters in Escherichia coli.Biochimie. 2002 Oct;84(10):981-96. doi: 10.1016/s0300-9084(02)00010-x. Biochimie. 2002. PMID: 12504278
-
Differential responses of Bacillus subtilis rRNA promoters to nutritional stress.J Bacteriol. 2011 Feb;193(3):723-33. doi: 10.1128/JB.00708-10. Epub 2010 Nov 19. J Bacteriol. 2011. PMID: 21097612 Free PMC article.
-
From microbial differentiation to ribosome engineering.Biosci Biotechnol Biochem. 2007 Jun;71(6):1373-86. doi: 10.1271/bbb.70007. Biosci Biotechnol Biochem. 2007. PMID: 17587668 Review.
-
Regulation of ribosomal RNA synthesis in E. coli: effects of the global regulator guanosine tetraphosphate (ppGpp).J Mol Microbiol Biotechnol. 2002 May;4(3):331-40. J Mol Microbiol Biotechnol. 2002. PMID: 11931566 Review.
Cited by
-
Diversity in (p)ppGpp metabolism and effectors.Curr Opin Microbiol. 2015 Apr;24:72-9. doi: 10.1016/j.mib.2015.01.012. Epub 2015 Jan 28. Curr Opin Microbiol. 2015. PMID: 25636134 Free PMC article. Review.
-
Distinct and contrasting transcription initiation patterns at Mycobacterium tuberculosis promoters.PLoS One. 2012;7(9):e43900. doi: 10.1371/journal.pone.0043900. Epub 2012 Sep 7. PLoS One. 2012. PMID: 22970148 Free PMC article.
-
The δ subunit of RNA polymerase is required for rapid changes in gene expression and competitive fitness of the cell.J Bacteriol. 2013 Jun;195(11):2603-11. doi: 10.1128/JB.00188-13. Epub 2013 Mar 29. J Bacteriol. 2013. PMID: 23543716 Free PMC article.
-
The ω Subunit Governs RNA Polymerase Stability and Transcriptional Specificity in Staphylococcus aureus.J Bacteriol. 2016 Dec 28;199(2):e00459-16. doi: 10.1128/JB.00459-16. Print 2017 Jan 15. J Bacteriol. 2016. PMID: 27799328 Free PMC article.
-
Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria.Sci Rep. 2019 Sep 23;9(1):13677. doi: 10.1038/s41598-019-50216-x. Sci Rep. 2019. PMID: 31548597 Free PMC article.
References
-
- Artsimovitch I, Patlan V, Sekine S, Vassylyeva MN, Hosaka T, Ochi K, Yokoyama S, Vassylyev DG (2004) Structural basis for transcription regulation by alarmone ppGpp. Cell 117: 299–310 - PubMed
-
- Barker MM (2001) PhD Thesis, University of Wisconsin-Madison
-
- Barker MM, Gaal T, Josaitis CA, Gourse RL (2001) Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol 305: 673–688 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

