The role of pneumolysin in pneumococcal pneumonia and meningitis
- PMID: 15498026
- PMCID: PMC1809205
- DOI: 10.1111/j.1365-2249.2004.02611.x
The role of pneumolysin in pneumococcal pneumonia and meningitis
Abstract
Diseases caused by Streptococcus pneumoniae include pneumonia, septicaemia and meningitis. All these are associated with high morbidity and mortality. The pneumococcus can colonize the nasopharynx, and this can be a prelude to bronchopneumonia and invasion of the vasculature space. Proliferation in the blood can result in a breach of the blood-brain barrier and entry into the cerebrospinal fluid (CSF) where the bacteria cause inflammation of the meningeal membranes resulting in meningitis. The infected host may develop septicaemia and/or meningitis secondary to bronchopneumonia. Also septicaemia is a common precursor of meningitis. The mechanisms surrounding the sequence of infection are unknown, but will be dependent on the properties of both the host and bacterium. Treatment of these diseases with antibiotics leads to clearance of the bacteria from the infected tissues, but the bacteriolytic nature of antibiotics leads to an acute release of bacterial toxins and thus after antibiotic therapy the patients can be left with organ-specific deficits. One of the main toxins released from pneumococci is the membrane pore forming toxin pneumolysin. Here we review the extensive studies on the role of pneumolysin in the pathogenesis of pneumococcal diseases.
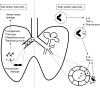
Figures
References
-
- Andrew PW, Mitchell TJ, Morgan PJ. Relationship of structure to function in pneumolysin. Microb Drug Resist. 1997;3:11–7. - PubMed
-
- Gilbert RJ, Jimenez JL, Chen S, et al. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell. 1999;97:647–55. - PubMed
-
- Boulnois GJ, Paton JC, Mitchell TJ, Andrew PW. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2611–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

