Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart
- PMID: 15498800
- PMCID: PMC1665392
- DOI: 10.1113/jphysiol.2004.077339
Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart
Abstract
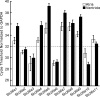
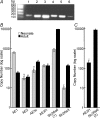
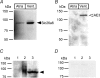
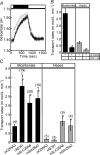
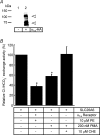
Bicarbonate facilitate more than 50% of pH recovery in the acidotic myocardium, and have roles in cardiac hypertrophy and steady-state pH regulation. To determine which bicarbonate transporters are responsible for this activity, we measured the expression levels of all known HCO3(-)-anion exchange proteins in mouse heart, by quantitative real time RT-PCR. Bicarbonate-anion exchangers are members of either the SLC4A or the SLC26A gene families. In neonatal and adult myocardium, AE1 (Slc4a1), AE2 (Slc4a2), AE3 (Slc4a3) (AE3fl and AE3c variants), Slc26a3 and Slc26a6 were expressed. Adult hearts expressed Slc26a3 and Slc4a1-3 mRNAs at similar levels, while Slc26a6 mRNA was about seven-fold higher than AE3, which was more abundant than any other. Immunohistochemistry revealed that Slc26a6 and AE3 are present in the plasma membrane of ventricular myocytes. Slc26a6 expression levels were higher in ventricle than atrium, whereas AE3 was detected only in ventricle. Cl(-)-HCO(3)(-) and Cl(-)-OH(-) exchange activity of SLC26A6 and AE3 were investigated in transfected HEK293 cells, using intracellular fluorescence measurements of 2',7'-bis (2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF), to monitor intracellular pH (pH(i)). Rates of pH(i) change were measured under HCO3(-)-containing (Cl(-)-HCO(3)(-)) or nominally HCO3(-)-free (Cl(-)-OH(-)) conditions. HCO3(-) fluxes were similar for cells expressing AE3fl, SLC26A6 or Slc26a3, suggesting that they have similar transport activity. However, only SLC26A6 and Slc26a3 functioned as Cl(-)-OH(-) exchangers. Activation of alpha-adrenergic receptors, which stimulates protein kinase C, inhibited SLC26A6 Cl(-)-HCO(3)(-) exchange activity. We conclude that Slc26a6 is the predominant Cl(-)-HCO(3)(-) and Cl(-)-OH(-) exchanger of the myocardium and that Slc26a6 is negatively regulated upon alpha-adrenergic stimulation.
Figures

References
-
- Alvarez BV, Fujinaga J, Casey JR. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ Res. 2001;89:1246–1253. - PubMed
-
- Camilion de Hurtado MC, Alvarez BV, Ennis IL, Cingolani HE. Stimulation of myocardial Na+-independent Cl−-HCO3− exchanger by angiotensin II is mediated by endogenous endothelin. Circ Res. 2000;86:622–627. - PubMed
-
- Camilion de Hurtado MC, Alvarez BV, Perez NG, Ennis IL, Cingolani HE. Angiotensin II activates Na+-independent Cl−-HCO3− exchange in ventricular myocardium. Circ Res. 1998;82:473–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

