3-hydroxy-3-methylglutaryl-CoA synthase intermediate complex observed in "real-time"
- PMID: 15498869
- PMCID: PMC534525
- DOI: 10.1073/pnas.0405809101
3-hydroxy-3-methylglutaryl-CoA synthase intermediate complex observed in "real-time"
Abstract
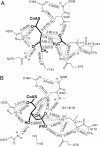
The formation of carbon-carbon bonds via an acyl-enzyme intermediate plays a central role in fatty acid, polyketide, and isoprenoid biosynthesis. Uniquely among condensing enzymes, 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase (HMGS) catalyzes the formation of a carbon-carbon bond by activating the methyl group of an acetylated cysteine. This reaction is essential in Gram-positive bacteria, and represents the first committed step in human cholesterol biosynthesis. Reaction kinetics, isotope exchange, and mass spectroscopy suggest surprisingly that HMGS is able to catalyze the "backwards" reaction in solution, where HMG-CoA is cleaved to form acetoacetyl-CoA (AcAc-CoA) and acetate. Here, we trap a complex of acetylated HMGS from Staphylococcus aureus and bound acetoacetyl-CoA by cryo-cooling enzyme crystals at three different times during the course of its back-reaction with its physiological product (HMG-CoA). This nonphysiological "backwards" reaction is used to understand the details of the physiological reaction with regards to individual residues involved in catalysis and substrate/product binding. The structures suggest that an active-site glutamic acid (Glu-79) acts as a general base both in the condensation between acetoacetyl-CoA and the acetylated enzyme, and the hydrolytic release of HMG-CoA from the enzyme. The ability to trap this enzyme-intermediate complex may suggest a role for protein dynamics and the interplay between protomers during the normal course of catalysis.
Figures



Comment in
-
An atomic-resolution mechanism of 3-hydroxy-3-methylglutaryl-CoA synthase.Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16399-400. doi: 10.1073/pnas.0407418101. Epub 2004 Nov 16. Proc Natl Acad Sci U S A. 2004. PMID: 15546978 Free PMC article. No abstract available.
References
-
- Teo, K. K. & Burton, J. R. (2002) Drugs 62, 1707–1715. - PubMed
-
- Campobasso, N., Patel, M., Wilding, E. I., Kallender, H., Rosenberg, M. & Gwynn, M. (2004) J. Biol. Chem. 279, 44883–44888. - PubMed
-
- Edwards, D. J., Marquez, B. L., Nogle, L. M., McPhail, K., Goeger, D. E., Roberts, M. A. & Gerwick, W. H. (2004) Chem. Biol. 11, 817–833. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

