Characterization of the B-cell inhibitory protein factor in Ixodes ricinus tick saliva: a potential role in enhanced Borrelia burgdoferi transmission
- PMID: 15500628
- PMCID: PMC1782588
- DOI: 10.1111/j.1365-2567.2004.01975.x
Characterization of the B-cell inhibitory protein factor in Ixodes ricinus tick saliva: a potential role in enhanced Borrelia burgdoferi transmission
Abstract
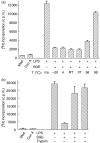
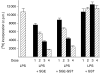
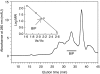
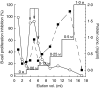
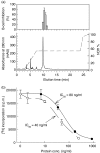
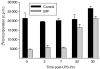
We recently described the inhibition of host B lymphocytes by Ixodes ricinus tick saliva. In this study, we characterized the factor responsible for this activity and examined the modulation of lipopolysaccharide (LPS)- and Borrelia burgdorferi outer surface protein (Osp)-induced proliferation of naive murine B lymphocytes by an enriched fraction of this factor. The B-lymphocyte inhibitory activity was destroyed by trypsin treatment, indicating that a proteinaceous factor was responsible for this activity. The removal of glutathione-S-transferase (GST) from tick salivary glands extracts (SGE) showed that this B-cell inhibitory protein (BIP) was not a GST. Gel filtration liquid chromatography indicated that BIP has a native molecular weight of approximately 18,000. An enrichment protocol, using a combination of anion-exchange and reverse-phase liquid chromatography, was established. BIP-enriched fractions did not suppress T-cell proliferation. Delayed addition of BIP-enriched fractions, up to 7 hr after LPS addition, inhibited the proliferation of isolated B cells. BIP-enriched fractions dramatically inhibited both OspA- and OspC-induced proliferation of isolated B cells. These results strongly suggest that BIP may facilitate B. burgdorferi transmission by preventing B-cell activation, and also highlights the potential of BIP as a therapeutic agent in B-cell maladies.
Figures

Similar articles
-
Interaction of primary mast cells with Borrelia burgdorferi (sensu stricto): role in transmission and dissemination in C57BL/6 mice.Parasit Vectors. 2017 Jun 27;10(1):313. doi: 10.1186/s13071-017-2243-0. Parasit Vectors. 2017. PMID: 28655322 Free PMC article.
-
Ixodes ricinus tick salivary gland extract inhibits IL-10 secretion and CD69 expression by mitogen-stimulated murine splenocytes and induces hyporesponsiveness in B lymphocytes.Parasite Immunol. 2003 Jan;25(1):27-37. doi: 10.1046/j.1365-3024.2003.00605.x. Parasite Immunol. 2003. PMID: 12753435
-
Whole-Chain Tick Saliva Proteins Presented on Hepatitis B Virus Capsid-Like Particles Induce High-Titered Antibodies with Neutralizing Potential.PLoS One. 2015 Sep 9;10(9):e0136180. doi: 10.1371/journal.pone.0136180. eCollection 2015. PLoS One. 2015. PMID: 26352137 Free PMC article.
-
Ménage à trois: Borrelia, dendritic cells, and tick saliva interactions.Trends Parasitol. 2014 Feb;30(2):95-103. doi: 10.1016/j.pt.2013.12.003. Epub 2013 Dec 30. Trends Parasitol. 2014. PMID: 24388562 Review.
-
Lyme borreliosis vaccination: the facts, the challenge, the future.Trends Parasitol. 2011 Jan;27(1):40-7. doi: 10.1016/j.pt.2010.06.006. Epub 2010 Jun 30. Trends Parasitol. 2011. PMID: 20594913 Review.
Cited by
-
Potential mechanisms implied in tick infection by arboviruses and their transmission to vertebrate hosts.Integr Zool. 2025 Mar;20(2):315-330. doi: 10.1111/1749-4877.12875. Epub 2024 Jul 17. Integr Zool. 2025. PMID: 39016029 Free PMC article. Review.
-
Ixodes scapularis saliva mitigates inflammatory cytokine secretion during Anaplasma phagocytophilum stimulation of immune cells.Parasit Vectors. 2012 Oct 10;5:229. doi: 10.1186/1756-3305-5-229. Parasit Vectors. 2012. PMID: 23050849 Free PMC article.
-
The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission.Front Cell Infect Microbiol. 2017 Jun 22;7:281. doi: 10.3389/fcimb.2017.00281. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28690983 Free PMC article. Review.
-
Lyme arthritis: current concepts and a change in paradigm.Clin Vaccine Immunol. 2008 Jan;15(1):21-34. doi: 10.1128/CVI.00330-07. Epub 2007 Nov 14. Clin Vaccine Immunol. 2008. PMID: 18003815 Free PMC article. Review. No abstract available.
-
Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display.PLoS One. 2011 Jan 5;6(1):e15926. doi: 10.1371/journal.pone.0015926. PLoS One. 2011. PMID: 21246036 Free PMC article.
References
-
- Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses. Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001;33:676–85. - PubMed
-
- Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–47. - PubMed
-
- Labuda M, Jones LD, Williams T, Nuttall PA. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Med Vet Entomol. 1993;7:193–6. - PubMed
-
- Zeidner NS, Schneider BS, Nuncio MS, Gern L, Piesman J. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J Parasitol. 2002;88:1276–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
