Factors involved in the inflammatory events of cervical ripening in humans
- PMID: 15500686
- PMCID: PMC534613
- DOI: 10.1186/1477-7827-2-74
Factors involved in the inflammatory events of cervical ripening in humans
Abstract
Background: Cervical ripening is an inflammatory reaction. The glucocorticoid receptor (GR) mediates glucocorticoid anti-inflammatory reactions, whereas nuclear factor (NF)kappaB is a key pro-inflammatory transcription factor. Prostaglandins as well as platelet activating factor (PAF) are inflammatory mediators. Inducible nitric oxide synthase (iNOS) regulates the level of nitric oxide (NO) in response to various inflammatory stimuli. We hypothesize that a changed biological response to glucocorticoids could be a mechanism regulating the inflammatory events resulting in cervical ripening.
Methods: We monitored GR and NFkappaB, prostaglandin synthases cyclooxygenase (COX)-1 and -2, iNOS, as well as the PAF-receptor (PAF-R) in the uterine cervix from term pregnant women (with unripe cervices) before the onset of labor (TP), immediately after parturition (PP), as compared to non-pregnant (NP), using immunohistochemistry and RT-PCR.
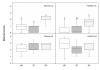
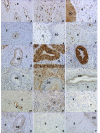
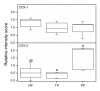
Results: The GR protein was detected by immunohistochemistry in the nuclei of stroma and squamous epithelium (SQ). Stromal GR staining was increased in TP as compared to the NP group and decreased again after parturition. GR staining in SQ was decreased after parturition as compared to term. NFkappaB was present in SQ and glandular epithelium (GE), stroma and vascular endothelium. Increased nuclear NFkappaB staining was observed postpartum as compared to term pregnancy in stroma and GE. Stromal immunostaining for COX-1 as well as COX-2 was increased in the TP and PP groups as compared to the NP, and GE displayed an intensely increased COX-2 immunostaining at term and postpartum. Stromal PAF-R immunostaining was highest at term, while it was greatly increased in GE postpartum. No difference in the immunostaining for iNOS was found between the groups. RT-PCR showed a predominance of GRalpha to GRbeta mRNA in cervical tissue. The COX-2 mRNA level was increased in the PP group as compared to the TP group.
Conclusions: There is a decrease in GR levels in human cervix at parturition. Concomitantly there is an increase of factors such as NFkappaB, PAF-R, COX-1 and COX-2, suggesting that they may participate in the sequence of events leading to the final cervical ripening.
Figures







References
-
- Ekman G, Malmström A, Uldbjerg N, Ulmsten U. Cervical collagen: an important regulator of cervical function in term labor. Obstet Gynecol. 1986;67:633–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

