Polyprotein cleavage mechanism of SARS CoV Mpro and chemical modification of the octapeptide
- PMID: 15501516
- PMCID: PMC7115412
- DOI: 10.1016/j.peptides.2004.06.018
Polyprotein cleavage mechanism of SARS CoV Mpro and chemical modification of the octapeptide
Abstract
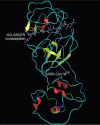
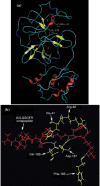
The cleavage mechanism of severe acute respiratory syndrome (SARS) coronavirus main proteinase (M(pro) or 3CL(pro)) for the octapeptide AVLQSGFR is studied using molecular mechanics (MM) and quantum mechanics (QM). The catalytic dyad His-41 and Cys-145 in the active pocket between domain I and II seem to polarize the pi-electron density of the peptide bond between Gln and Ser in the octapeptide, leading to an increase of positive charge on C(CO) of Gln and negative charge on N(NH) of Ser. The possibility of enhancing the chemical bond between Gln and Ser based on the "distorted key" theory [Anal. Biochem. 233 (1996) 1] is examined. The scissile peptide bond between Gln and Ser is found to be solidified through "hybrid peptide bond" by changing the carbonyl group CO of Gln to CH(2) or CF(2). This leads to a break of the pi-bond system for the peptide bond, making the octapeptide (AVLQSGFR) a "distorted key" and a potential starting system for the design of anti SARS drugs.
Figures
References
-
- Althaus I.W., Chou J.J., Gonzales A.J., Diebel M.R., Chou K.C., Kezdy F.J., et al. Kinetic studies with the nonnucleoside HIV-1 reverse transcriptase inhibitor U-88204E. Biochemistry. 1993;32:6548–6554. - PubMed
-
- Althaus I.W., Chou J.J., Gonzales A.J., Diebel M.R., Chou K.C., Kezdy F.J., et al. Steady-state kinetic studies with the non-nucleoside HIV-1 reverse transcriptase inhibitor U-87201E. J. Biol. Chem. 1993;268:6119–6124. - PubMed
-
- Althaus I.W., Chou J.J., Gonzales A.J., Diebel M.R., Chou K.C., Kezdy F.J., et al. Steady-state kinetic studies with the polysulfonate U-9843, an HIV reverse transcriptase inhibitor. Experientia. 1994;50:23–28. - PubMed
-
- Althaus I.W., Chou J.J., Gonzales A.J., Diebel M.R., Chou K.C., Kezdy F.J., et al. Kinetic studies with the non-nucleoside HIV-1 reverse transcriptase inhibitor U-90152E. Biochem. Pharmacol. 1994;47:2017–2028. - PubMed
-
- Althaus I.W., Chou K.C., Franks K.M., Diebel M.R., Kezdy F.J., Romero D.L., et al. The benzylthio-pyrididine U-31355 is a potent inhibitor of HIV-1 reverse transcriptase. Biochem. Pharmacol. 1996;51:743–750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

