Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation
- PMID: 15501746
- PMCID: PMC523030
- DOI: 10.1128/IAI.72.11.6211-6220.2004
Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation
Abstract
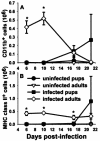
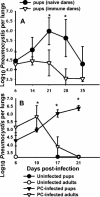
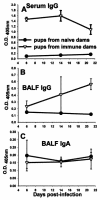
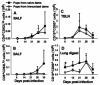
Pneumocystis carinii is an opportunistic fungal pathogen that causes life-threatening pneumonia in immunocompromised individuals. Infants appear to be particularly susceptible to infection with Pneumocystis. We have previously shown that there is a significant delay in clearance of the organisms from the lungs of neonatal mice compared to adults. Since alveolar macrophages are the effector cells responsible for killing and clearance of Pneumocystis, we have examined alveolar macrophage activity in neonatal mice. We found that alveolar macrophage activation is delayed about 1 week in Pneumocystis-infected neonates compared to adults. Opsonization of the organism by Pneumocystis-specific antibody resulted in increased clearance of the organism in neonatal mice; however, there was decreased expression of activation markers on neonatal alveolar macrophages and reduced levels of cytokines associated with macrophage activation. Mice born to immunized dams had significant amounts of Pneumocystis-specific immunoglobulin G in their lungs and serum at day 7 postinfection, whereas mice born to naive dams had merely detectable levels. This difference correlated with enhanced Pneumocystis clearance in mice born to immunized dams. The increase in specific antibody, however, did not result in significant inflammation in the lungs, as no differences in numbers of activated CD4+ cells were observed. Furthermore, there was no difference in cytokine or chemokine concentrations in the lungs of pups born to immune compared to naive dams. These findings indicate that specific antibody plays an important role in Pneumocystis clearance from lungs of infected neonates; moreover, this process occurs without inducing inflammation in the lungs.
Figures

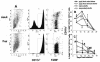


References
-
- Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20:330-335. - PubMed
-
- Adkins, B., and R.-Q. Du. 1998. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J. Immunol. 160:4217-4224. - PubMed
-
- Adkins, B., and K. Hamilton. 1992. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J. Immunol. 149:3448-3455. - PubMed
-
- Berclaz, P. Y., Y. Shibata, J. A. Whitsett, and B. C. Trapnell. 2002. GM-CSF, via PU.1, regulates alveolar macrophage FcγR-mediated phagocytosis and the IL-18/IFN-γ-mediated molecular connection between innate and adaptive immunity in the lung. Blood 100:4193-4200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

