Strain-specific humoral response to a polymorphic malaria vaccine
- PMID: 15501757
- PMCID: PMC523016
- DOI: 10.1128/IAI.72.11.6300-6305.2004
Strain-specific humoral response to a polymorphic malaria vaccine
Abstract
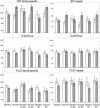
The 3D7 form of the merozoite surface protein 2 (MSP2) of Plasmodium falciparum was one of three subunits of the malaria vaccine Combination B that were tested in a phase I/IIb double-blind randomized placebo-controlled trial, which was undertaken with 120 Papua New Guinean children of 5 to 9 years of age. Because only one variant of the highly polymorphic MSP2 was used for vaccination, we examined whether the elicited response was directed against conserved or strain-specific epitopes. Postvaccination (week 12) titers of antibody against recombinantly expressed individual domains of MSP2 were measured by enzyme-linked immunosorbent assay and compared to baseline values. We found that vaccination with the 3D7 form of MSP2 induced a significant strain-specific humoral response directed against the repetitive and semiconserved family-specific part. The conserved N- and C-terminal domains were not immunogenic. Titers of antibody against the alternate FC27 family-specific domain showed a tendency to increase in vaccinated children, but there was no increase in antibodies against FC27-type 32-mer repeats. These results indicate that vaccination with one MSP2 variant mainly induced a strain-specific response, which can explain the selective effect of vaccination with combination B on the genotypes of breakthrough parasites. These findings support the inclusion of both family-specific domains (3D7 and FC27) in an improved vaccine formulation.
Figures

Similar articles
-
Effect of the malaria vaccine Combination B on merozoite surface antigen 2 diversity.Infect Genet Evol. 2007 Jan;7(1):44-51. doi: 10.1016/j.meegid.2006.03.006. Epub 2006 May 2. Infect Genet Evol. 2007. PMID: 16647307 Clinical Trial.
-
Evaluation of two long synthetic merozoite surface protein 2 peptides as malaria vaccine candidates.Vaccine. 2009 May 5;27(20):2653-61. doi: 10.1016/j.vaccine.2009.02.081. Epub 2009 Mar 6. Vaccine. 2009. PMID: 19428875
-
A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea.J Infect Dis. 2002 Mar 15;185(6):820-7. doi: 10.1086/339342. Epub 2002 Feb 14. J Infect Dis. 2002. PMID: 11920300 Clinical Trial.
-
Emerging rules for subunit-based, multiantigenic, multistage chemically synthesized vaccines.Acc Chem Res. 2008 Mar;41(3):377-86. doi: 10.1021/ar700120t. Epub 2008 Feb 12. Acc Chem Res. 2008. PMID: 18266328 Review.
-
Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research.Vaccine. 2005 Mar 18;23(17-18):2243-50. doi: 10.1016/j.vaccine.2005.01.142. Vaccine. 2005. PMID: 15755604 Review.
Cited by
-
High-throughput identification of the predominant malaria parasite clone in complex blood stage infections using a multi-SNP molecular haplotyping assay.Am J Trop Med Hyg. 2007 Jan;76(1):12-9. Am J Trop Med Hyg. 2007. PMID: 17255222 Free PMC article.
-
Mixed allele malaria vaccines: host protection and within-host selection.Vaccine. 2008 Nov 11;26(48):6099-107. doi: 10.1016/j.vaccine.2008.09.004. Epub 2008 Sep 18. Vaccine. 2008. PMID: 18804509 Free PMC article.
-
Vaccines for preventing malaria (blood-stage).Cochrane Database Syst Rev. 2006 Oct 18;2006(4):CD006199. doi: 10.1002/14651858.CD006199. Cochrane Database Syst Rev. 2006. PMID: 17054281 Free PMC article.
-
Host-Malaria Parasite Interactions and Impacts on Mutual Evolution.Front Cell Infect Microbiol. 2020 Oct 27;10:587933. doi: 10.3389/fcimb.2020.587933. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194831 Free PMC article. Review.
-
Antigenic characterization of an intrinsically unstructured protein, Plasmodium falciparum merozoite surface protein 2.Infect Immun. 2012 Dec;80(12):4177-85. doi: 10.1128/IAI.00665-12. Epub 2012 Sep 10. Infect Immun. 2012. PMID: 22966050 Free PMC article.
References
-
- Alonso, P. L., T. Smith, J. R. Schellenberg, H. Masanja, S. Mwankusye, H. Urassa, I. Bastos de Azevedo, J. Chongela, S. Kobero, C. Menendez, N. Hurt, M. C. Thomas, E. Lyimo, N. A. Weiss, R. Hayes, A. Y. Kitua, M. C., Lopez, W. L., Kilama, T. Teuscher, and M. Tanner. 1994. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet 344:1175-1181. - PubMed
-
- Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J., Conway, W. H., Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A., Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, T. Doherty, and RTS,S Malaria Vaccine Trial Team. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. - PubMed
-
- Bradley-Moore, A. M., B. M. Greenwood, A. K. Bradley, A. Bartlett, D. E. Bidwell, A. Voller, J. Craske, B. R. Kirkwood, and H. M. Gilles. 1985. Malaria chemoprophylaxis with chloroquine in young Nigerian children. II. Effect on the immune response to vaccination. Ann. Trop. Med. Parasitol. 79:563-573. - PubMed
-
- Bygbjerg, I. C., N. Odum, and T. G. Theander. 1986. Effect of pyrimethamine and sulphadoxine on human lymphocyte proliferation. Trans. R. Soc. Trop. Med. Hyg. 80:295-300. - PubMed
-
- Coulibaly, D., D. A. Diallo, M. A. Thera, A. Dicko, A. B. Guindo, A. K. Kone, Y. Cissoko, S. Coulibaly, A. Djimde, K. Lyke, O. K. Doumbo, and C. V. Plowe. 2002. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am. J. Trop. Med. Hyg. 67:604-610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

