The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection
- PMID: 15501762
- PMCID: PMC523032
- DOI: 10.1128/IAI.72.11.6330-6340.2004
The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection
Abstract
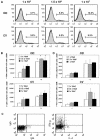
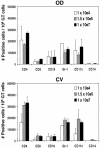
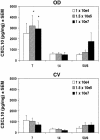
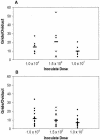
Murine vaginal infection with the obligate intracellular bacterium Chlamydia muridarum is commonly used as a model for ascending Chlamydia infections of the human female genital tract. Gamma interferon-producing Th1 cells, in concert with other mononuclear infiltrates, primarily mediate antichlamydial immunity. However, many factors modify this response, including the bacterial load. To investigate the manner in which the inoculating dose of C. muridarum modulates a genital infection, we measured innate and adaptive cell numbers, CD4+ lymphocyte cytokine profile, chemokine expression, course of infection, and pathological sequelae in genital tracts of BALB/c mice infected with doses of C. muridarum ranging from 10(4) to 10(7) inclusion-forming units. We found that the influx of both innate and adaptive immune cells responded similarly in the lower genital tract (cervical-vaginal tissues) and upper genital tract (oviduct tissues) to increasing inoculating doses. However, cells expressing the innate markers Gr-1 and CD11c were affected to a greater degree by increasing dose than lymphocytes of the adaptive immune response (Th1, CD4+, CD8+, CD19+), resulting in a change in the balance of innate and adaptive cell numbers to favor innate cells at higher infecting doses. Surprisingly, we detected greater numbers of viable chlamydiae in the oviducts at lower inoculating doses, and the number of organisms appeared to directly correlate with hydrosalpinx formation after both primary infection and repeat infection. Taken together, these data suggest that innate immune cells contribute to control of ascending infection.
Figures

Similar articles
-
Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production.Infect Immun. 2007 Feb;75(2):666-76. doi: 10.1128/IAI.01280-06. Epub 2006 Nov 21. Infect Immun. 2007. PMID: 17118987 Free PMC article.
-
Effects of inoculating dose on the kinetics of Chlamydia muridarum genital infection in female mice.Immunol Cell Biol. 2009 May-Jun;87(4):337-43. doi: 10.1038/icb.2009.3. Epub 2009 Feb 10. Immunol Cell Biol. 2009. PMID: 19204735
-
Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease.J Immunol. 2007 Sep 15;179(6):4027-34. doi: 10.4049/jimmunol.179.6.4027. J Immunol. 2007. PMID: 17785841
-
Immunopathogenesis of genital Chlamydia infection: insights from mouse models.Pathog Dis. 2021 Mar 31;79(4):ftab012. doi: 10.1093/femspd/ftab012. Pathog Dis. 2021. PMID: 33538819 Free PMC article. Review.
-
T cell responses to Chlamydia.Pathog Dis. 2021 Mar 31;79(4):ftab014. doi: 10.1093/femspd/ftab014. Pathog Dis. 2021. PMID: 33693620 Free PMC article. Review.
Cited by
-
Waddlia chondrophila induces systemic infection, organ pathology, and elicits Th1-associated humoral immunity in a murine model of genital infection.Front Cell Infect Microbiol. 2015 Nov 4;5:76. doi: 10.3389/fcimb.2015.00076. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26583077 Free PMC article.
-
The effect of infectious dose on humoral and cellular immune responses in Chlamydophila caviae primary ocular infection.PLoS One. 2017 Jul 5;12(7):e0180551. doi: 10.1371/journal.pone.0180551. eCollection 2017. PLoS One. 2017. PMID: 28678871 Free PMC article.
-
Modulation of Immune Response to Chlamydia muridarum by Host miR-135a.Front Cell Infect Microbiol. 2021 Apr 13;11:638058. doi: 10.3389/fcimb.2021.638058. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33928045 Free PMC article.
-
Genital Infiltrations of CD4+ and CD8+ T Lymphocytes, IgA+ and IgG+ Plasma Cells and Intra-Mucosal Lymphoid Follicles Associate With Protection Against Genital Chlamydia trachomatis Infection in Minipigs Intramuscularly Immunized With UV-Inactivated Bacteria Adjuvanted With CAF01.Front Microbiol. 2019 Feb 8;10:197. doi: 10.3389/fmicb.2019.00197. eCollection 2019. Front Microbiol. 2019. PMID: 30800114 Free PMC article.
-
Estrogen Receptor Alpha (ESR1)-Dependent Regulation of the Mouse Oviductal Transcriptome.PLoS One. 2016 Jan 25;11(1):e0147685. doi: 10.1371/journal.pone.0147685. eCollection 2016. PLoS One. 2016. PMID: 26808832 Free PMC article.
References
-
- Anttila, T., P. Saikku, P. Koskela, A. Bloigu, J. Dillner, I. Ikaheimo, E. Jellum, M. Lehtinen, P. Lenner, T. Hakulinen, A. Narvanen, E. Pukkala, S. Thoresen, L. Youngman, and J. Paavonen. 2001. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 285:47-51. - PubMed
-
- Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1994. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 2:94-98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

