Glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, inhibits the progression of group B streptococcal arthritis
- PMID: 15501766
- PMCID: PMC523051
- DOI: 10.1128/IAI.72.11.6367-6372.2004
Glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, inhibits the progression of group B streptococcal arthritis
Abstract
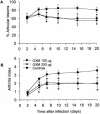
Glucuronoxylomannan (GXM), the principal constituent of the Cryptococcus neoformans capsule, modulates the inflammatory response of human monocytes in vitro. Here we examine the efficacy of GXM as a novel anti-inflammatory compound for use against experimental septic arthritis. Arthritis was induced in mice by the intravenous injection of 8 x 10(6) CFU of type IV group B streptococcus (GBS). GXM was administered intravenously in different doses (50, 100, or 200 microg/mouse) 1 day before and 1 day after bacterial inoculation. GXM treatment markedly decreased the incidence and severity of articular lesions. Histological findings showed limited periarticular inflammation in the joints of GXM-treated mice, confirming the clinical observations. The amelioration of arthritis was associated with a significant reduction in the local production of interleukin-6 (IL-6), IL-1beta, macrophage inflammatory protein 1alpha (MIP-1alpha), and MIP-2 and an increase in systemic IL-10 levels. Moreover, peritoneal macrophages derived from GXM-treated mice and stimulated in vitro with heat-inactivated GBS showed a similar pattern of cytokine production. The present study provides evidence for the modulation of the inflammatory response by GXM in vivo and suggests a potential therapeutic use for this compound in pathologies involving inflammatory processes.
Figures



Similar articles
-
Role of interleukin-18 in experimental group B streptococcal arthritis.Arthritis Rheum. 2004 Jun;50(6):2005-13. doi: 10.1002/art.20014. Arthritis Rheum. 2004. PMID: 15188378
-
Regulatory role of interleukin-10 in experimental group B streptococcal arthritis.Infect Immun. 2002 Jun;70(6):2862-8. doi: 10.1128/IAI.70.6.2862-2868.2002. Infect Immun. 2002. PMID: 12010973 Free PMC article.
-
IL-4 deficiency decreases mortality but increases severity of arthritis in experimental group B Streptococcus infection.Mediators Inflamm. 2009;2009:394021. doi: 10.1155/2009/394021. Epub 2009 Jul 7. Mediators Inflamm. 2009. PMID: 19606256 Free PMC article.
-
Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model.Vaccine. 1996 Jun;14(9):841-4. doi: 10.1016/0264-410x(95)00256-z. Vaccine. 1996. PMID: 8843625 Review.
-
The cellular responses induced by the capsular polysaccharide of Cryptococcus neoformans differ depending on the presence or absence of specific protective antibodies.Curr Mol Med. 2005 Jun;5(4):413-20. doi: 10.2174/1566524054022585. Curr Mol Med. 2005. PMID: 15977997 Review.
Cited by
-
Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions.Infect Immun. 2010 Apr;78(4):1601-9. doi: 10.1128/IAI.01171-09. Epub 2010 Feb 9. Infect Immun. 2010. PMID: 20145096 Free PMC article.
-
Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan.PLoS Pathog. 2006 Nov;2(11):e120. doi: 10.1371/journal.ppat.0020120. PLoS Pathog. 2006. PMID: 17096589 Free PMC article.
-
Capsular Material of Cryptococcus neoformans: Virulence and Much More.Mycopathologia. 2012 Feb 8. doi: 10.1007/s11046-011-9513-8. Online ahead of print. Mycopathologia. 2012. PMID: 22314939
-
A purified capsular polysaccharide markedly inhibits inflammatory response during endotoxic shock.Infect Immun. 2013 Jan;81(1):90-8. doi: 10.1128/IAI.00553-12. Epub 2012 Oct 22. Infect Immun. 2013. PMID: 23090956 Free PMC article.
-
Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses.mBio. 2013 Jan 15;4(1):e00522-12. doi: 10.1128/mBio.00522-12. mBio. 2013. PMID: 23322637 Free PMC article.
References
-
- Arend, W. P., and J. M. Dayer. 1990. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 33:305-315. - PubMed
-
- Arend, W. P., and J. M. Dayer. 1995. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 38:151-160. - PubMed
-
- Baker, C. J., and M. S. Edwards. 1995. Group B streptococcal infections, p. 980-1054. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 4th ed. W. B. Saunders, Philadelphia, Pa.
-
- Bennett, J. E., H. F. Hasenclaver, and B. S. Tynes. 1964. Detection of cryptococcal polysaccharide in serum and spinal fluid: value in diagnosis and prognosis. Trans. Assoc. Am. Physician 77:145-150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

