Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines
- PMID: 15501772
- PMCID: PMC523039
- DOI: 10.1128/IAI.72.11.6418-6425.2004
Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines
Abstract
Plasmids represent a powerful tool to rapidly introduce genes into bacteria and help them reach high expression levels. In vaccine development, with live vaccine vectors, this allows greater flexibility and the ability to induce larger antigen amounts through multiple gene copies. However, plasmid retention often requires antibiotic resistance markers, the presence of which has been discouraged in clinical applications by the Food and Drug Administration. Therefore, we developed a Listeria monocytogenes-Escherichia coli shuttle plasmid that is retained by complementation of D-alanine racemase-deficient mutant strains both in vitro and in vivo. Our technology potentially allows the production of antibiotic resistance marker-free DNA vaccines as well as bacterial vaccine vectors devoid of engineered antibiotic resistances. As a proof of concept, we applied the D-alanine racemase complementation system to our Listeria cancer vaccine platform. With a transplantable tumor model, we compared the efficacy of the new Listeria vector to that of an established vector containing a conventional plasmid carrying a tumor-specific antigen. Both vaccine vector systems resulted in long-term regression of established tumors, with no significant difference between them. Thus, the Listeria vaccine vector presented here potentially complies with Food and Drug Administration regulations and could be developed further for clinical use.
Figures
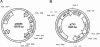

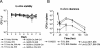
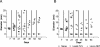
Similar articles
-
Prophylactic and therapeutic efficacy of an attenuated Listeria monocytogenes-based vaccine delivering HPV16 E7 in a mouse model.Int J Mol Med. 2012 Dec;30(6):1335-42. doi: 10.3892/ijmm.2012.1136. Epub 2012 Sep 20. Int J Mol Med. 2012. PMID: 23027427
-
In vivo bactofection: listeria can function as a DNA-cancer vaccine.DNA Cell Biol. 2006 Mar;25(3):142-51. doi: 10.1089/dna.2006.25.142. DNA Cell Biol. 2006. PMID: 16569193
-
Stable integration vector for nutrient broth-based selection of attenuated Listeria monocytogenes strains with recombinant antigen expression.Clin Vaccine Immunol. 2008 Sep;15(9):1414-9. doi: 10.1128/CVI.00208-08. Epub 2008 Jul 23. Clin Vaccine Immunol. 2008. PMID: 18650400 Free PMC article.
-
Live attenuated bacteria as vectors to deliver plasmid DNA vaccines.Curr Opin Mol Ther. 2003 Feb;5(1):10-9. Curr Opin Mol Ther. 2003. PMID: 12669465 Review.
-
Listeria-based vaccines for cancer treatment.Curr Opin Mol Ther. 2005 Oct;7(5):454-60. Curr Opin Mol Ther. 2005. PMID: 16248280 Review.
Cited by
-
Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis.Pathogens. 2023 Nov 23;12(12):1380. doi: 10.3390/pathogens12121380. Pathogens. 2023. PMID: 38133265 Free PMC article. Review.
-
Lm-LLO-Based Immunotherapies and HPV-Associated Disease.J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. Epub 2012 Feb 2. J Oncol. 2012. PMID: 22481930 Free PMC article.
-
Brucella abortus strain RB51 leucine auxotroph as an environmentally safe vaccine for plasmid maintenance and antigen overexpression.Appl Environ Microbiol. 2008 Nov;74(22):7051-5. doi: 10.1128/AEM.01511-08. Epub 2008 Oct 3. Appl Environ Microbiol. 2008. PMID: 18836016 Free PMC article.
-
ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen.Hum Vaccin Immunother. 2014;10(11):3190-5. doi: 10.4161/hv.34378. Hum Vaccin Immunother. 2014. PMID: 25483687 Free PMC article. Review.
-
Comparison of different live vaccine strategies in vivo for delivery of protein antigen or antigen-encoding DNA and mRNA by virulence-attenuated Listeria monocytogenes.Infect Immun. 2006 Jul;74(7):3946-57. doi: 10.1128/IAI.00112-06. Infect Immun. 2006. PMID: 16790768 Free PMC article.
References
-
- Ausubel, K., R. Brent, and R. E. Kingston. 1997. Current protocols in molecular biology. Wiley Interscience, New York, N.Y.
-
- Behnke, D., M. S. Gilmore, and J. J. Ferretti. 1981. Plasmid pGB301, a new multiple resistance streptococcal cloning vehicle and its use in cloning of a gentamicin/kanamycin resistance determinant. Mol. Gen. Genet. 182:414-421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

