RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants
- PMID: 15501774
- PMCID: PMC523033
- DOI: 10.1128/IAI.72.11.6433-6445.2004
RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants
Abstract
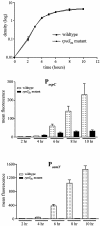
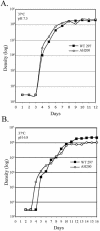
Borrelia burgdorferi, the Lyme disease spirochete, undergoes dramatic changes in antigenic composition as it cycles between its arthropod and mammalian hosts. A growing body of evidence suggests that these changes reflect, at least in part, the need for spirochetes to adapt to the physiological stresses imposed by abrupt changes in environmental conditions and nutrient availability. In many microorganisms, global responses are mediated by master regulators such as alternative sigma factors, with Escherichia coli RpoS (sigmaS) serving as a prototype. The importance of this transcriptional activator in other bacteria, coupled with the report by Hubner et al. (A. Hubner, X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard, Proc. Natl. Acad. Sci. USA 98:12724-12729, 2001) demonstrating that the borrelial RpoS ortholog controls expression of OspC and decorin-binding protein A (DbpA), prompted us to examine more closely the roles of RpoS-dependent and -independent differential gene expression in physiological adaptation by the Lyme disease spirochete. We observed that B. burgdorferi rpoS (rpoSBb) was induced following temperature shift and transcript levels were further enhanced by reduced pH (pH 6.8). Using quantitative real-time reverse transcription-PCR (RT-PCR), we demonstrated that, in contrast to its ortholog (rpoSEc) in Escherichia coli, rpoSBb was expressed at significant levels in B. burgdorferi throughout all phases of growth following temperature shift. By comparing a B. burgdorferi strain 297 rpoSBb mutant to its wild-type counterpart, we determined that RpoSBb was not required for survival following exposure to a wide range of environmental stresses (i.e., temperature shift, serum starvation, increased osmolality, reactive oxygen intermediates, and increased or reduced oxygen tension), although the mutant was more sensitive to extremes of pH. While B. burgdorferi strains lacking RpoS were able to survive within intraperitoneal dialysis membrane chambers at a level equivalent to that of the wild type, they were avirulent in mice. Lastly, RT-PCR analysis of the ospE-ospF-elp paralogous lipoprotein families complements earlier findings that many temperature-inducible borrelial loci are controlled in an RpoSBb-independent manner. Together, these data point to fundamental differences between the role(s) of RpoS in B. burgdorferi and that in E. coli. Rather than functioning as a master regulator, RpoSBb appears to serve as a stress-responsive activator of a subset of virulence determinants that, together with the RpoS-independent, differentially expressed regulon, encompass the spirochete's genetic programs required for mammalian host adaptation.
Figures


References
-
- Akins, D. R., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homolog. Mol. Microbiol. 18:507-520. - PubMed
-
- Alban, P. S., P. W. Johnson, and D. R. Nelson. 2000. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 146:119-127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

