Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes
- PMID: 15501783
- PMCID: PMC523031
- DOI: 10.1128/IAI.72.11.6519-6527.2004
Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes
Abstract
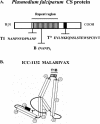
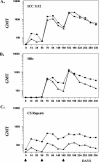
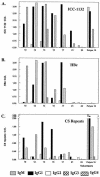
We report the first phase I trial to assess the safety and immunogenicity of a malaria vaccine candidate, ICC-1132 (Malarivax), composed of a modified hepatitis B virus core protein (HBc) containing minimal epitopes of the Plasmodium falciparum circumsporozoite (CS) protein. When expressed in Escherichia coli, the recombinant ICC-1132 protein forms virus-like particles that were found to be highly immunogenic in preclinical studies of mice and monkeys. Twenty healthy adult volunteers received a 20- or a 50-microg dose of alum-adsorbed ICC-1132 administered intramuscularly at 0, 2, and 6 months. The majority of volunteers in the group receiving the 50-microg dose developed antibodies to CS repeats as well as to HBc. Malaria-specific T cells that secreted gamma interferon were also detected after a single immunization with ICC-1132-alum. These studies support ICC-1132 as a promising malaria vaccine candidate for further clinical testing using more-potent adjuvant formulations and confirm the potential of modified HBc virus-like particles as a delivery platform for vaccines against other human pathogens.
Figures
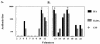
References
-
- Ballou, W. R., S. L. Hoffman, J. A. Sherwood, M. R. Hollingdale, F. A. Neva, W. T. Hockmeyer, D. M. Gordon, I. Schneider, R. A. Wirtz, J. F. Young, et al. 1987. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet 1:1277-1281. - PubMed
-
- Ballou, W. R., J. Rothbard, R. A. Wirtz, D. M. Gordon, J. S. Williams, R. W. Gore, I. Schneider, M. R. Hollingdale, R. L. Beaudoin, W. L. Maloy, et al. 1985. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science 228:996-999. - PubMed
-
- Birkett, A., K. Lyons, A. Schmidt, D. Boyd, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. Nardin. 2002. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infect. Immun. 70:6860-6870. - PMC - PubMed
-
- Birkett, A., B. Thornton, D. Milich, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. H. Nardin. 2001. Hepatitis B virus core antigen particles containing minimal T and B cell epitopes of Plasmodium falciparum CS protein elicit high levels of malaria specific immune responses in mice and non-human primates. Am. J. Trop. Med. Hyg. 65:258.
-
- Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

