The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha
- PMID: 15501784
- PMCID: PMC523053
- DOI: 10.1128/IAI.72.11.6528-6537.2004
The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha
Abstract
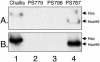
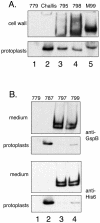
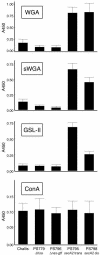
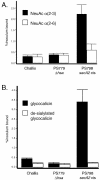
Platelet binding by Streptococcus gordonii strain M99 is dependent on expression of the cell wall-anchored glycoprotein GspB. This large cell surface protein is exported from the M99 cytoplasm via a dedicated transport system that includes SecA2 and SecY2. GspB is highly similar to Hsa, a protein expressed by S. gordonii Challis that has been characterized as a sialic acid binding hemagglutinin. In this study, we compared the contribution of GspB and Hsa to the adherence of S. gordonii to selected glycoproteins. Our results indicate that GspB can mediate binding to a variety of sialylated glycoproteins. GspB facilitates binding to carbohydrates bearing sialic acid in either alpha(2-3) or alpha(2-6) linkages, with a slight preference for alpha(2-3) linkages. Furthermore, GspB readily mediates binding to sialic acid residues on immobilized glycocalicin, the extracellular portion of the platelet membrane glycoprotein (GP) Ibalpha (the ligand binding subunit of the platelet von Willebrand factor receptor complex GPIb-IX-V). Although Hsa is required for the binding of S. gordonii Challis to sialic acid, most of the Hsa expressed by Challis is retained in the cytoplasm. The deficiency in export is due, at least in part, to a nonsense mutation in secA2. Hsa export can be enhanced by complementation with secA2 from M99, which also results in significantly greater binding to sialylated glycoproteins, including glycocalicin. The combined results indicate that GspB and Hsa contribute similar binding capabilities to M99 and Challis, respectively, but there may be subtle differences in the preferred epitopes to which these adhesins bind.
Figures


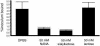

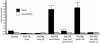
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
-
- Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. - PubMed
-
- Berndt, M. C., C. Gregory, A. Kabral, H. Zola, D. Fournier, and P. A. Castaldi. 1985. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur. J. Biochem. 151:637-649. - PubMed
-
- Bryan, E. M., T. Bae, M. Kleerebezem, and G. M. Dunny. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183-190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

