Enhanced immunogenicity in the murine airway mucosa with an attenuated Salmonella live vaccine expressing OprF-OprI from Pseudomonas aeruginosa
- PMID: 15501786
- PMCID: PMC523058
- DOI: 10.1128/IAI.72.11.6546-6553.2004
Enhanced immunogenicity in the murine airway mucosa with an attenuated Salmonella live vaccine expressing OprF-OprI from Pseudomonas aeruginosa
Abstract
We constructed an oral live vaccine based on the attenuated aroA mutant Salmonella enterica serovar Typhimurium strain SL3261 expressing outer membrane proteins F and I (OprF-OprI) from Pseudomonas aeruginosa and investigated it in a mouse model. Strains with in vivo inducible protein expression with the PpacC promoter showed good infection rates and immunogenicity but failed to engender detectable antibodies in the lung. However, a systemic booster vaccination following an oral primary immunization yielded high immunoglobulin A (IgA) and IgG antibody levels in both upper and lower airways superior to conventional systemic or mucosal booster vaccination alone. In addition, the proportion of IgG1 and IgG2a antibodies suggested that the systemic booster does not alter the more TH1-like type of response induced by the oral Salmonella primary vaccination. We conclude that an oral primary systemic booster vaccination strategy with an appropriate mucosal vector may be advantageous in diseases with the risk of P. aeruginosa airway infection, such as cystic fibrosis.
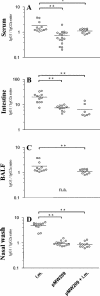
Figures
Similar articles
-
Mechanistic research holds promise for bacterial vaccines and phage therapies for Pseudomonas aeruginosa.Drug Des Devel Ther. 2019 Mar 20;13:909-924. doi: 10.2147/DDDT.S189847. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 30936684 Free PMC article. Review.
-
Assessment of pulmonary antibodies with induced sputum and bronchoalveolar lavage induced by nasal vaccination against Pseudomonas aeruginosa: a clinical phase I/II study.Respir Res. 2007 Aug 5;8(1):57. doi: 10.1186/1465-9921-8-57. Respir Res. 2007. PMID: 17683588 Free PMC article. Clinical Trial.
-
Salmonella Typhimurium strain expressing OprF-OprI protects mice against fatal infection by Pseudomonas aeruginosa.Microbiol Immunol. 2015 Sep;59(9):533-44. doi: 10.1111/1348-0421.12291. Microbiol Immunol. 2015. PMID: 26249788
-
Systemic, nasal and oral live vaccines against Pseudomonas aeruginosa: a clinical trial of immunogenicity in lower airways of human volunteers.Vaccine. 2010 Jan 8;28(3):707-13. doi: 10.1016/j.vaccine.2009.10.080. Epub 2009 Nov 1. Vaccine. 2010. PMID: 19887136
-
Killed whole bacterial cells, a mucosal delivery system for the induction of immunity in the respiratory tract and middle ear: an overview.Vaccine. 1999 Mar 26;17(13-14):1775-81. doi: 10.1016/s0264-410x(98)00441-1. Vaccine. 1999. PMID: 10194839 Review.
Cited by
-
Understanding Pseudomonas aeruginosa-Host Interactions: The Ongoing Quest for an Efficacious Vaccine.Cells. 2020 Dec 5;9(12):2617. doi: 10.3390/cells9122617. Cells. 2020. PMID: 33291484 Free PMC article. Review.
-
Antibody isotype analysis of malaria-nematode co-infection: problems and solutions associated with cross-reactivity.BMC Immunol. 2010 Feb 17;11:6. doi: 10.1186/1471-2172-11-6. BMC Immunol. 2010. PMID: 20163714 Free PMC article.
-
Mechanistic research holds promise for bacterial vaccines and phage therapies for Pseudomonas aeruginosa.Drug Des Devel Ther. 2019 Mar 20;13:909-924. doi: 10.2147/DDDT.S189847. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 30936684 Free PMC article. Review.
-
Beneficial effects of TLR-2/6 ligation in pulmonary bacterial infection and immunization with Pseudomonas aeruginosa.Inflammation. 2010 Feb;33(1):58-64. doi: 10.1007/s10753-009-9158-7. Inflammation. 2010. PMID: 19844782
-
Bacterial Ghosts of Pseudomonas aeruginosa as a Promising Candidate Vaccine and Its Application in Diabetic Rats.Vaccines (Basel). 2022 Jun 7;10(6):910. doi: 10.3390/vaccines10060910. Vaccines (Basel). 2022. PMID: 35746518 Free PMC article.
References
-
- Allen, J. S., G. Dougan, and R. A. Strugnell. 2000. Kinetics of the mucosal antibody secreting cell response and evidence of specific lymphocyte migration to the lung after oral immunisation with attenuated S. enterica var. typhimurium. FEMS Immunol. Med. Microbiol. 27:275-281. - PubMed
-
- Baud, D., J. Benyacoub, V. Revaz, M. Kok, F. Ponci, M. Bobst, R. Curtiss III, P. De Grandi, and D. Nardelli-Haefliger. 2004. Immunogenicity against human papillomavirus type 16 virus-like particles is strongly enhanced by the PhoPc phenotype in Salmonella enterica serovar Typhimurium. Infect. Immun. 72:750-756. - PMC - PubMed
-
- Boucher, R. C. 2004. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 23:146-158. - PubMed
-
- Brennan, A. L., and D. M. Geddes. 2002. Cystic fibrosis. Curr. Opin. Infect. Dis. 15:175-182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

