Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells
- PMID: 15501810
- PMCID: PMC523038
- DOI: 10.1128/IAI.72.11.6717-6721.2004
Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells
Abstract
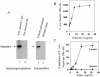
Human galectin-3 binds to the surface of Trypanosoma cruzi trypomastigotes and human coronary artery smooth muscle (CASM) cells. CASM cells express galectin-3 on their surface and secrete it. Exogenous galectin-3 increased the binding of T. cruzi to CASM cells. Trypanosome binding to CASM cells was enhanced when either T. cruzi or CASM cells were preincubated with galectin-3. Cells stably transfected with galectin-3 antisense show a dramatic decrease in galectin-3 expression and very little T. cruzi adhesion to cells. The addition of galectin-3 to these cells restores their initial capacity to bind to trypanosomes. Thus, host galectin-3 expression is required for T. cruzi adhesion to human cells and exogenous galectin-3 enhances this process, leading to parasite entry.
Figures




Similar articles
-
Gene network analysis during early infection of human coronary artery smooth muscle cells by Trypanosoma cruzi and Its gp83 ligand.Chem Biodivers. 2010 May;7(5):1051-64. doi: 10.1002/cbdv.200900320. Chem Biodivers. 2010. PMID: 20491065 Free PMC article.
-
Recruitment of galectin-3 during cell invasion and intracellular trafficking of Trypanosoma cruzi extracellular amastigotes.Glycobiology. 2014 Feb;24(2):179-84. doi: 10.1093/glycob/cwt097. Epub 2013 Nov 12. Glycobiology. 2014. PMID: 24225883
-
Trypanosoma cruzi infection induces proliferation of vascular smooth muscle cells.Infect Immun. 2006 Jan;74(1):152-9. doi: 10.1128/IAI.74.1.152-159.2006. Infect Immun. 2006. PMID: 16368968 Free PMC article.
-
Novel mechanism that Trypanosoma cruzi uses to adhere to the extracellular matrix mediated by human galectin-3.FEBS Lett. 2000 Mar 31;470(3):305-8. doi: 10.1016/s0014-5793(00)01347-8. FEBS Lett. 2000. PMID: 10745086
-
Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity.Parasitol Int. 2008 Jun;57(2):105-9. doi: 10.1016/j.parint.2007.12.008. Epub 2007 Dec 23. Parasitol Int. 2008. PMID: 18234547 Review.
Cited by
-
A Carbohydrate Moiety of Secreted Stage-Specific Glycoprotein 4 Participates in Host Cell Invasion by Trypanosoma cruzi Extracellular Amastigotes.Front Microbiol. 2018 Apr 10;9:693. doi: 10.3389/fmicb.2018.00693. eCollection 2018. Front Microbiol. 2018. PMID: 29692765 Free PMC article.
-
Utilization of Galectins by Pathogens for Infection.Front Immunol. 2020 Aug 19;11:1877. doi: 10.3389/fimmu.2020.01877. eCollection 2020. Front Immunol. 2020. PMID: 32973776 Free PMC article. Review.
-
Cell signaling during Trypanosoma cruzi invasion.Front Immunol. 2012 Nov 28;3:361. doi: 10.3389/fimmu.2012.00361. eCollection 2012. Front Immunol. 2012. PMID: 23230440 Free PMC article.
-
Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis.Cell Microbiol. 2008 Oct;10(10):2078-90. doi: 10.1111/j.1462-5822.2008.01190.x. Epub 2008 Jul 10. Cell Microbiol. 2008. PMID: 18637021 Free PMC article.
-
The roles of galectins in parasitic infections.Acta Trop. 2018 Jan;177:97-104. doi: 10.1016/j.actatropica.2017.09.027. Epub 2017 Oct 3. Acta Trop. 2018. PMID: 28986248 Free PMC article. Review.
References
-
- Acosta-Rodriguez, E. V., C. L. Montes, C. C. Motran, E. I. Zuniga, F.-T. Liu, G. A. Rabinovich, and A. Gruppi. 2004. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 172:493-502. - PubMed
-
- Frigeri, L. G., and F.-T. Liu. 1992. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J. Immunol. 148:861-869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

