Mechanical properties of Xenopus egg cytoplasmic extracts
- PMID: 15501931
- PMCID: PMC1305045
- DOI: 10.1529/biophysj.104.048025
Mechanical properties of Xenopus egg cytoplasmic extracts
Abstract
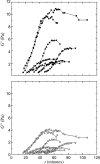
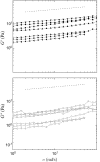
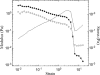
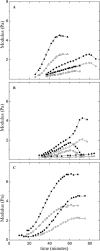
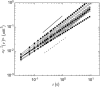
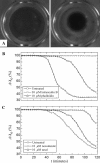
Cytoplasmic extracts prepared from Xenopus laevis eggs are used for the reconstitution of a wide range of processes in cell biology, and offer a unique environment in which to investigate the role of cytoplasmic mechanics without the complication of preorganized cellular structures. As a step toward understanding the mechanical properties of this system, we have characterized the rheology of crude interphase extracts. At macroscopic length scales, the extract forms a soft viscoelastic solid. Using a conventional mechanical rheometer, we measure the elastic modulus to be in the range of 2-10 Pa, and loss modulus in the range of 0.5-5 Pa. Using pharmacological and immunological disruption methods, we establish that actin filaments and microtubules cooperate to give mechanical strength, whereas the intermediate filament cytokeratin does not contribute to viscoelasticity. At microscopic length scales smaller than the average network mesh size, the response is predominantly viscous. We use multiple particle tracking methods to measure the thermal fluctuations of 1 microm embedded tracer particles, and measure the viscosity to be approximately 20 mPa-s. We explore the impact of rheology on actin-dependent cytoplasmic contraction, and find that although microtubules modulate contractile forces in vitro, their interactions are not purely mechanical.
Figures

References
-
- Alberts, B., A. Johnson, J. Lewis, M. Raff, K. Roberts, and P. Walter. 2002. Molecular Biology of the Cell. Garland, New York.
-
- Boal, D. 2002. Mechanics of the Cell. Cambridge University Press, Cambridge.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

