Effects of phenylalanine substitutions in gramicidin A on the kinetics of channel formation in vesicles and channel structure in SDS micelles
- PMID: 15501932
- PMCID: PMC1305000
- DOI: 10.1529/biophysj.104.047456
Effects of phenylalanine substitutions in gramicidin A on the kinetics of channel formation in vesicles and channel structure in SDS micelles
Abstract
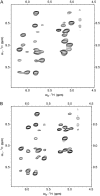
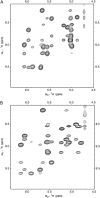
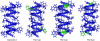
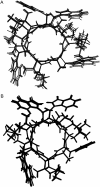
The common occurrence of Trp residues at the aqueous-lipid interface region of transmembrane channels is thought to be indicative of its importance for insertion and stabilization of the channel in membranes. To further investigate the effects of Trp-->Phe substitution on the structure and function of the gramicidin channel, four analogs of gramicidin A have been synthesized in which the tryptophan residues at positions 9, 11, 13, and 15 are sequentially replaced with phenylalanine. The three-dimensional structure of each viable analog has been determined using a combination of two-dimensional NMR techniques and distance geometry-simulated annealing structure calculations. These phenylalanine analogs adopt a homodimer motif, consisting of two beta6.3 helices joined by six hydrogen bonds at their NH2-termini. The replacement of the tryptophan residues does not have a significant effect on the backbone structure of the channels when compared to native gramicidin A, and only small effects are seen on side-chain conformations. Single-channel conductance measurements have shown that the conductance and lifetime of the channels are significantly affected by the replacement of the tryptophan residues (Wallace, 2000; Becker et al., 1991). The variation in conductance appears to be caused by the sequential removal of a tryptophan dipole, thereby removing the ion-dipole interaction at the channel entrance and at the ion binding site. Channel lifetime variations appear to be related to changing side chain-lipid interactions. This is supported by data relating to transport and incorporation kinetics.
Figures

Similar articles
-
The structure, cation binding, transport, and conductance of Gly15-gramicidin A incorporated into SDS micelles and PC/PG vesicles.Biochemistry. 2003 Feb 18;42(6):1401-9. doi: 10.1021/bi0204286. Biochemistry. 2003. PMID: 12578352
-
Effects of glycine substitutions on the structure and function of gramicidin a channels.Biochemistry. 2006 Nov 28;45(47):14012-20. doi: 10.1021/bi061560z. Biochemistry. 2006. PMID: 17115696
-
Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration.Biochemistry. 1991 Sep 10;30(36):8830-9. doi: 10.1021/bi00100a015. Biochemistry. 1991. PMID: 1716152
-
Design and characterization of gramicidin channels with side chain or backbone mutations.Novartis Found Symp. 1999;225:44-55; discussion 55-61. doi: 10.1002/9780470515716.ch4. Novartis Found Symp. 1999. PMID: 10472047 Review.
-
Correlations of structure, dynamics and function in the gramicidin channel by solid-state NMR spectroscopy.Novartis Found Symp. 1999;225:4-16; discussion 16-22. Novartis Found Symp. 1999. PMID: 10472044 Review.
Cited by
-
Shorter Antibacterial Peptide Having High Selectivity for E. coli Membranes and Low Potential for Inducing Resistance.Microorganisms. 2020 Jun 8;8(6):867. doi: 10.3390/microorganisms8060867. Microorganisms. 2020. PMID: 32521823 Free PMC article.
-
How to Combat Gram-Negative Bacteria Using Antimicrobial Peptides: A Challenge or an Unattainable Goal?Antibiotics (Basel). 2021 Dec 7;10(12):1499. doi: 10.3390/antibiotics10121499. Antibiotics (Basel). 2021. PMID: 34943713 Free PMC article. Review.
-
Novel formulations for antimicrobial peptides.Int J Mol Sci. 2014 Oct 9;15(10):18040-83. doi: 10.3390/ijms151018040. Int J Mol Sci. 2014. PMID: 25302615 Free PMC article. Review.
References
-
- Andersen, O. S., D. V. Greathouse, L. L. Providence, M. D. Becker, and R. E. Koeppe, Ii. 1998. Importance of tryptophan dipoles for protein function: 5-fluorination of tryptophans in gramicidin A channels. J. Am. Chem. Soc. 120:5142–5146.
-
- Arseniev, A. S., I. L. Barsukov, V. F. Bystrov, A. L. Lomize, and A. Ovchinnikov Yu. 1985. Proton NMR study of gramicidin A transmembrane ion channel. Head-to-head right-handed, single-stranded helices. FEBS Lett. 186:168–174. - PubMed
-
- Becker, M. D., D. V. Greathouse, R. E. Koeppe II, and O. S. Andersen. 1991. Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration. Biochemistry. 30:8830–8839. - PubMed
-
- Braunschweiler, L., and R. R. Ernst. 1983. Coherence transfer by isotropic mixing: Application to proton correlation spectroscopy. J. Magn. Reson. 53:521–528.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

