Effects of phenylalanine substitutions in gramicidin A on the kinetics of channel formation in vesicles and channel structure in SDS micelles
- PMID: 15501932
- PMCID: PMC1305000
- DOI: 10.1529/biophysj.104.047456
Effects of phenylalanine substitutions in gramicidin A on the kinetics of channel formation in vesicles and channel structure in SDS micelles
Abstract
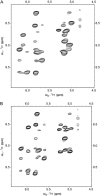
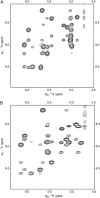
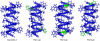
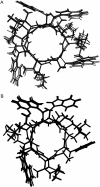
The common occurrence of Trp residues at the aqueous-lipid interface region of transmembrane channels is thought to be indicative of its importance for insertion and stabilization of the channel in membranes. To further investigate the effects of Trp-->Phe substitution on the structure and function of the gramicidin channel, four analogs of gramicidin A have been synthesized in which the tryptophan residues at positions 9, 11, 13, and 15 are sequentially replaced with phenylalanine. The three-dimensional structure of each viable analog has been determined using a combination of two-dimensional NMR techniques and distance geometry-simulated annealing structure calculations. These phenylalanine analogs adopt a homodimer motif, consisting of two beta6.3 helices joined by six hydrogen bonds at their NH2-termini. The replacement of the tryptophan residues does not have a significant effect on the backbone structure of the channels when compared to native gramicidin A, and only small effects are seen on side-chain conformations. Single-channel conductance measurements have shown that the conductance and lifetime of the channels are significantly affected by the replacement of the tryptophan residues (Wallace, 2000; Becker et al., 1991). The variation in conductance appears to be caused by the sequential removal of a tryptophan dipole, thereby removing the ion-dipole interaction at the channel entrance and at the ion binding site. Channel lifetime variations appear to be related to changing side chain-lipid interactions. This is supported by data relating to transport and incorporation kinetics.
Figures

References
-
- Andersen, O. S., D. V. Greathouse, L. L. Providence, M. D. Becker, and R. E. Koeppe, Ii. 1998. Importance of tryptophan dipoles for protein function: 5-fluorination of tryptophans in gramicidin A channels. J. Am. Chem. Soc. 120:5142–5146.
-
- Arseniev, A. S., I. L. Barsukov, V. F. Bystrov, A. L. Lomize, and A. Ovchinnikov Yu. 1985. Proton NMR study of gramicidin A transmembrane ion channel. Head-to-head right-handed, single-stranded helices. FEBS Lett. 186:168–174. - PubMed
-
- Becker, M. D., D. V. Greathouse, R. E. Koeppe II, and O. S. Andersen. 1991. Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration. Biochemistry. 30:8830–8839. - PubMed
-
- Braunschweiler, L., and R. R. Ernst. 1983. Coherence transfer by isotropic mixing: Application to proton correlation spectroscopy. J. Magn. Reson. 53:521–528.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

